C2H6 Wedge Drawing
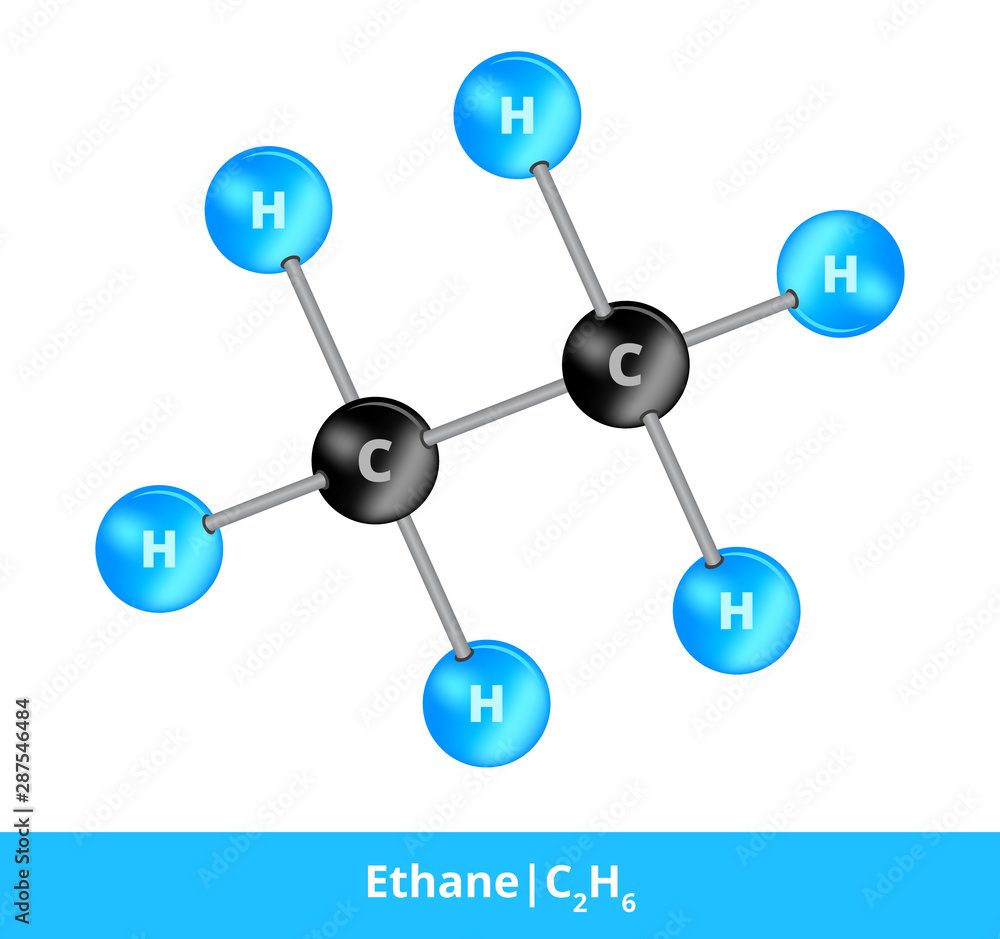
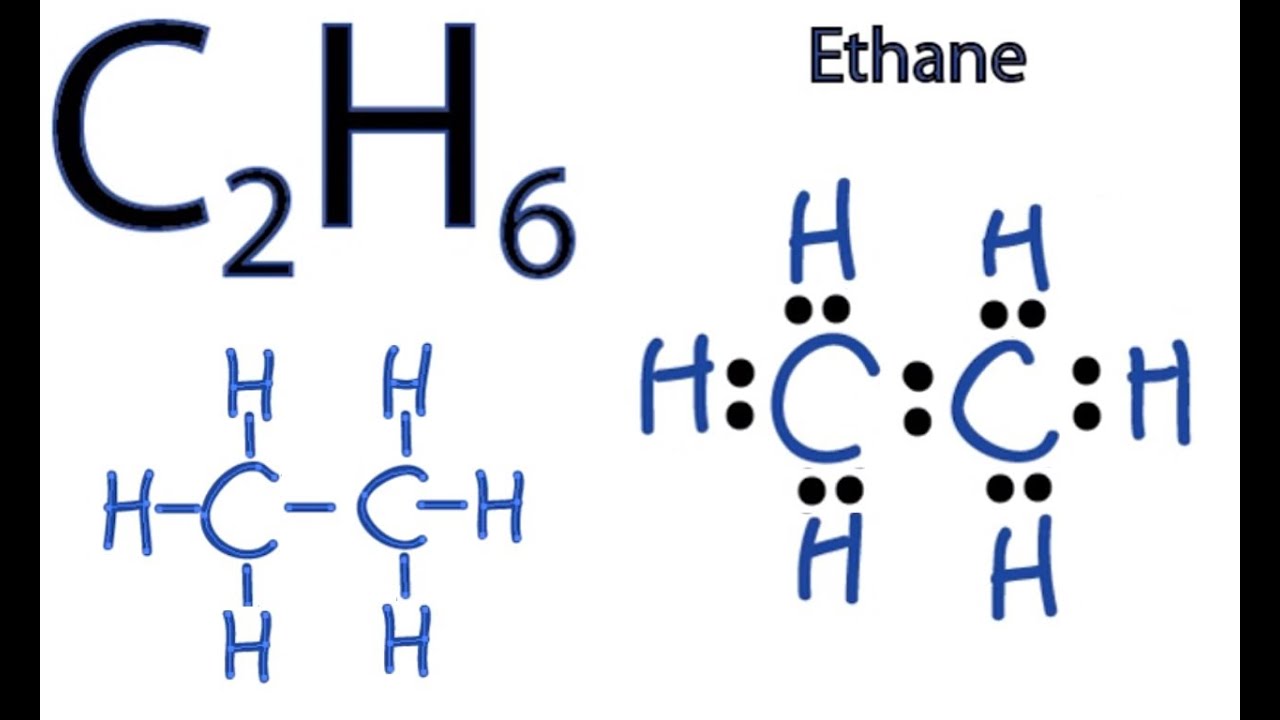
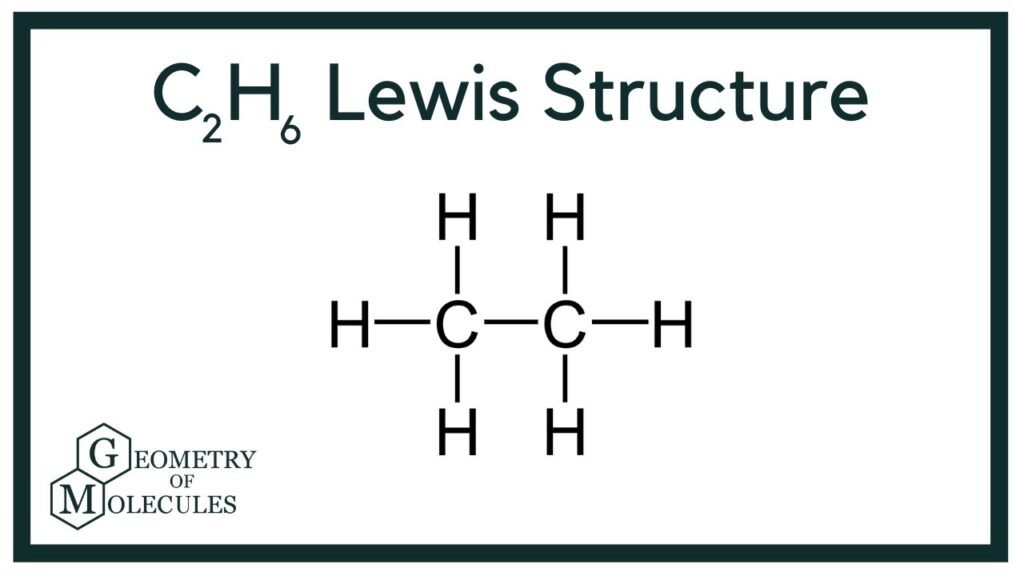
C2H6 Wedge Drawing - In ethane, we have two carbon atoms and 6 hydrogen atoms and hence, the total number of valence electron are (2 x. Build propane (c 3 h 8). At stp it is colorless, odorless gas. If there are equivalent resonance structures, draw all of them. This compound is one of the simplest hydrocarbons to exist having a single bond between. They follow the duet rule (2 electrons). Staggered ethane contains a main c 3 rotation axis with 3 c 2 rotation axis perpendicular to the c 3 axis, with 3 σ d planes. Web draw lewis structure (s) for the ethane molecule ( c2h6 ). Again, that's due to stability. As a suggestion, they seem to be most effective when the similar pairs.
It is a colorless and odorless molecule that exists as a gas at the standard room temperature. Staggered ethane contains a main c 3 rotation axis with 3 c 2 rotation axis perpendicular to the c 3 axis, with 3 σ d planes. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Make a wedge drawing of the following molecules. Web c2h6 lewis structure: Web at room temperature, there's enough energy for the staggered conformation to turn into the eclipsed. Ethane is an organic compound with a chemical formula of c2h6. Make a wedge drawing of the following molecules. As a suggestion, they seem to be most effective when the similar pairs. Note that hydrogen only needs two valence electrons to.
Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. Web you often draw them to suit your own purposes. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. Determining the total number of valence electrons in the molecule. Three are two ways to draw the lewis structure for c2h6o. Click the symmetry operations above to view them in 3d. Staggered ethane contains a main c 3 rotation axis with 3 c 2 rotation axis perpendicular to the c 3 axis, with 3 σ d planes. The exception, of course, being the hydrogen's. If there are equivalent resonance structures, draw all of them. Complete the octet (or duplet) on outside atoms.
C2H6 Molecular Geometry / Shape and Bond Angles YouTube
Click the symmetry operations above to view them in 3d. Whenever you see a compound made of carbon and hydrogen that ends in ane that means it will only have single bonds. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw these 2 stereoisomers of 1,3 cyclohexanediol as chairs. The carbons.
How to Draw the Lewis Dot Structure for C2H6 Ethane YouTube
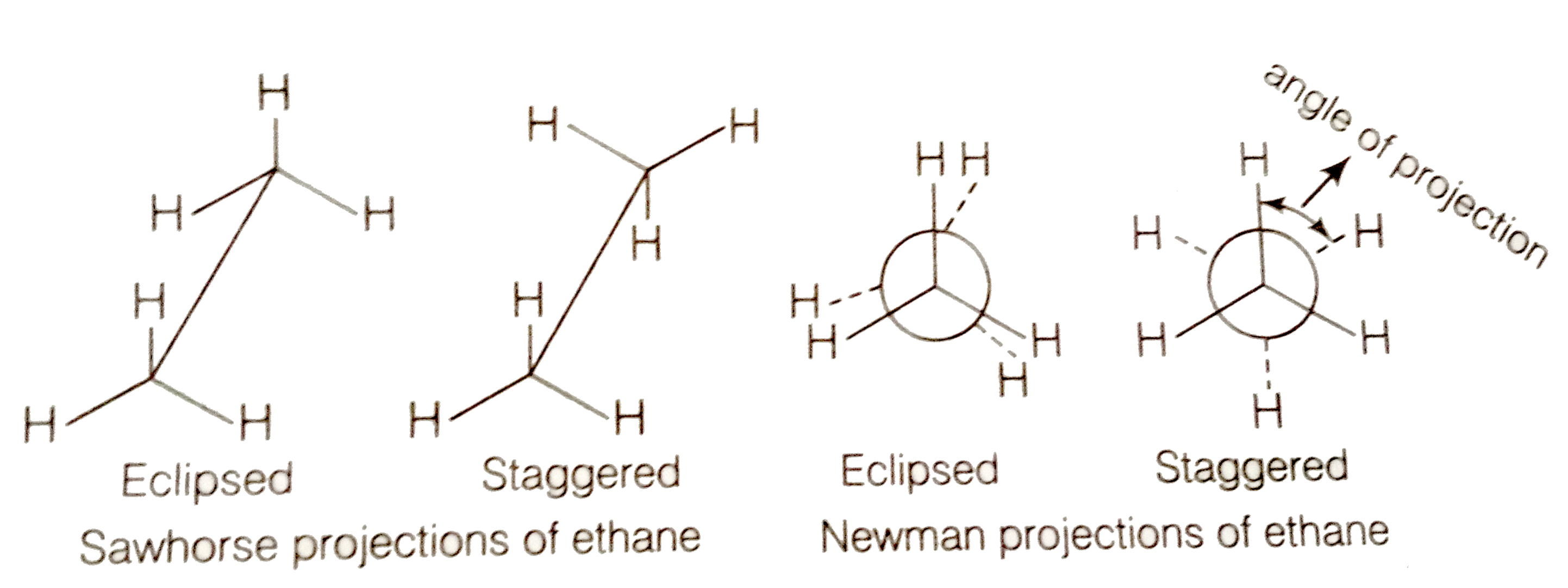
The exception, of course, being the hydrogen's. Draw these 2 stereoisomers of 1,3 cyclohexanediol as chairs. At stp it is colorless, odorless gas. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal. Web staggered vs eclipsed conformations of ethane, or why newman projections are awesome.
Electron Dot Diagram For Methane Wiring Diagram
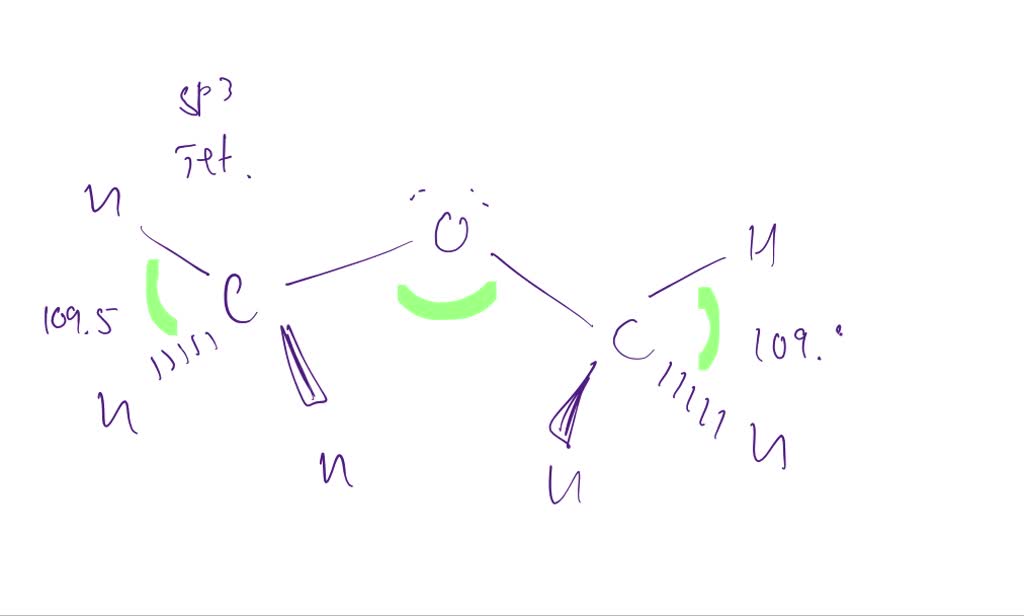
Web the hatched line is read as this atom is farther away from us. Ethane is a saturated hydrocarbon found in natural gas. Again, that's due to stability. Separate sketches with the ↔ symbol. Click the symmetry operations above to view them in 3d.
Draw Newman and sawhorse projections for the eclipsed and staggered co
Dnd | dnh | dn pointgroups. Web lewis dot of ethane. Staggered ethane contains a main c 3 rotation axis with 3 c 2 rotation axis perpendicular to the c 3 axis, with 3 σ d planes. If there are equivalent resonance structures, draw all of them. Again, that's due to stability.
Vector ballandstick model of chemical substance. Icon of ethane
Note that hydrogen only needs two valence electrons to. Here’s how you can easily draw the c 2 h 6 lewis structure step by step: Whenever you see a compound made of carbon and hydrogen that ends in ane that means it will only have single bonds. If there are equivalent resonance structures, draw all of them. Since all the.
C2H6 Lewis Structure How to Draw the Dot Structure for C2H6 YouTube
They follow the duet rule (2 electrons). Web this wedge and dash drawing represents one conformation of ethane. Web staggered vs eclipsed conformations of ethane, or why newman projections are awesome. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The carbons on the bottom of the zigzag will have.
What Is C2h6 Lewis Structure?
Web you often draw them to suit your own purposes. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Here’s how you can easily draw the c 2 h 6 lewis structure step by step: Separate sketches with the ↔ symbol. They follow the duet rule (2 electrons).
C2h6 Molecule
Web c2h6 lewis structure: Note that hydrogen only needs two valence electrons to. Web this wedge and dash drawing represents one conformation of ethane. Ethane (eclipsed) pointgroup flow chart. Web in the c2h6 lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms.
Lewis Dot Diagram For C2h6
70 more lewis dot structures. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Make a wedge drawing of the following molecules. Do not include overall ion charges or formal charges in your drawing. Make a wedge drawing of the following molecules.
SOLVEDUsing lines, solid wedges, and dashed wedges, draw the three
Staggered ethane contains a main c 3 rotation axis with 3 c 2 rotation axis perpendicular to the c 3 axis, with 3 σ d planes. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. Note that hydrogen only needs two valence electrons to. Determining the total number of valence electrons in.
Here’s How You Can Easily Draw The C 2 H 6 Lewis Structure Step By Step:
Separate sketches with the ↔ symbol. Do not include overall ion charges or formal charges in your drawing. Web lewis dot of ethane. 70 more lewis dot structures.
Since All The Atoms Are In Either Period 1 Or 2, This Molecule Will Adhere To The Octet Rule.
Dnd | dnh | dn pointgroups. If there are equivalent resonance structures, draw all of them. Draw these 2 stereoisomers of 1,3 cyclohexanediol as chairs. Determining the total number of valence electrons in the molecule.
Draw 2 Stereoisomers Of 1,3 Cyclohexanediol With Wedges And Dashes.
With c 2 h 6 there are only single bonds. If it's polar, indicate the direction of the polarity on your wedge drawing. They follow the duet rule (2 electrons). Web at room temperature, there's enough energy for the staggered conformation to turn into the eclipsed.
Web C2H6 Lewis Structure:
The valence electron for carbon (1s22s22p2) and hydrogen (1s1) is 4 and 1, respectively. Note that hydrogen only needs two valence electrons to. Web this wedge and dash drawing represents one conformation of ethane. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom.