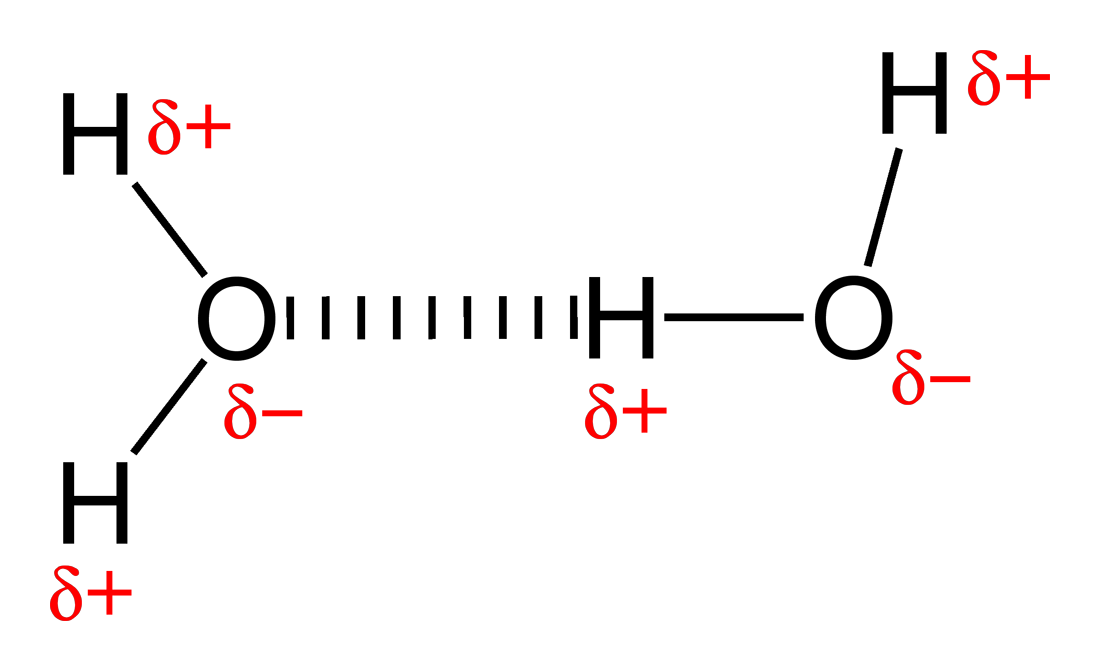
Can Ch3Oh Form Hydrogen Bonds
Can Ch3Oh Form Hydrogen Bonds - Web $\begingroup$ i don't know whether this can be proven or disproven. Hence ch₃oh forms hydrogen bonding. Two with the hydrogen atoms and two with the with the oxygen atoms. So yes, we can have hydrogen bonding between one h2o molecule and one hcl molecule, in which case the o molecule in h2o forms a hydrogen bond with the h from hcl. Hydrogen bonds are not as stable as covalences. Again ch3 + carbocation is very unstable. The hydrogen bonds with oxygen thus forming a hydrogen bond. One consequence of this is that, even though dimethyl ether is a gas at room temperature, you can prepare a. It cannot form hydrogen bonds with other ch₃br molecules. Web science chemistry chemistry questions and answers which molecule or molecules can form hydrogen bonds with water?
Web $\begingroup$ i don't know whether this can be proven or disproven. Web science chemistry chemistry questions and answers which molecule or molecules can form hydrogen bonds with water? The hydrogen bonds with oxygen thus forming a hydrogen bond. It cannot form hydrogen bonds with other ch₃br molecules. In principle, the oxygen atom of ch3oh has two unused doublets. Ch3ch2ch3 ch4 ch3coch3 ch3oh which salt would lower the freezing point of a solvent the most? Ch₃br has no n, o, or f atoms, and it has no h atoms attached to n, o, or f. Which molecule or molecules can form hydrogen bonds with water? Web can ch3oh form a hydrogen bond? Web answer (1 of 10):
3.om c6h1206 1.om cacl2 1.25m nacl 1.25m na2so4. One consequence of this is that, even though dimethyl ether is a gas at room temperature, you can prepare a. Hydrogen bonds are not as stable as covalences. In principle, the oxygen atom of ch3oh has two unused doublets. Web science chemistry chemistry questions and answers which molecule or molecules can form hydrogen bonds with water? Web chemistry questions and answers. Again ch3 + carbocation is very unstable. Ch3ch2ch3 ch4 ch3coch3 ch3oh which salt would lower the freezing point of a solvent the most? Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web can ch3oh form a hydrogen bond?
The Curious Wavefunction A bond by any other name... How the simple
Which of the following molecules can form hydrogen bonds in samples of the pure substance? Hydrogen bonds are not as stable as covalences. Two with the hydrogen atoms and two with the with the oxygen atoms. Web $\begingroup$ i don't know whether this can be proven or disproven. Hence ch₃oh forms hydrogen bonding.
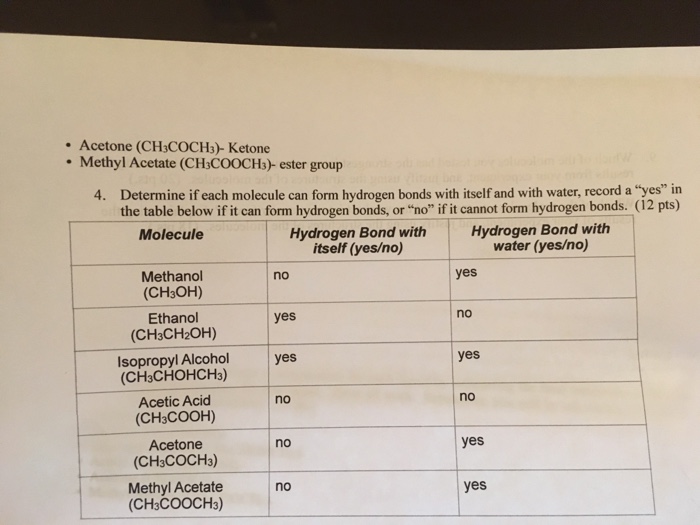
Solved If my chart on yes or no could be looked over for
It cannot form hydrogen bonds with other ch₃br molecules. Web answer (1 of 10): Which of the following molecules can form hydrogen bonds in samples of the pure substance? Web science chemistry chemistry questions and answers which molecule or molecules can form hydrogen bonds with water? It can form hydrogen bonds with other ch₃oh molecules.
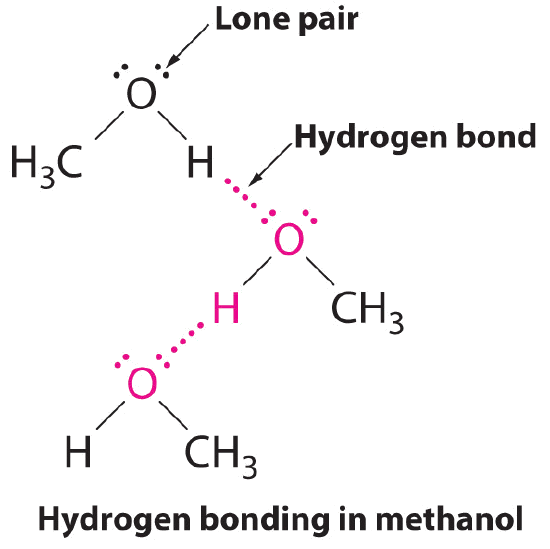
Which drawing below best represents hydrogen bonding methanol, ch3oh
It can form hydrogen bonds with other ch₃oh molecules. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web chemistry questions and answers. 3.om c6h1206 1.om cacl2 1.25m nacl 1.25m na2so4. Again ch3 + carbocation is very unstable.
Diagram Of Water Molecules Hydrogen Bonding
It can form hydrogen bonds with other ch₃oh molecules. 3.om c6h1206 1.om cacl2 1.25m nacl 1.25m na2so4. Web science chemistry chemistry questions and answers which molecule or molecules can form hydrogen bonds with water? Ch₃br has no n, o, or f atoms, and it has no h atoms attached to n, o, or f. The hydrogen bonds with oxygen thus.
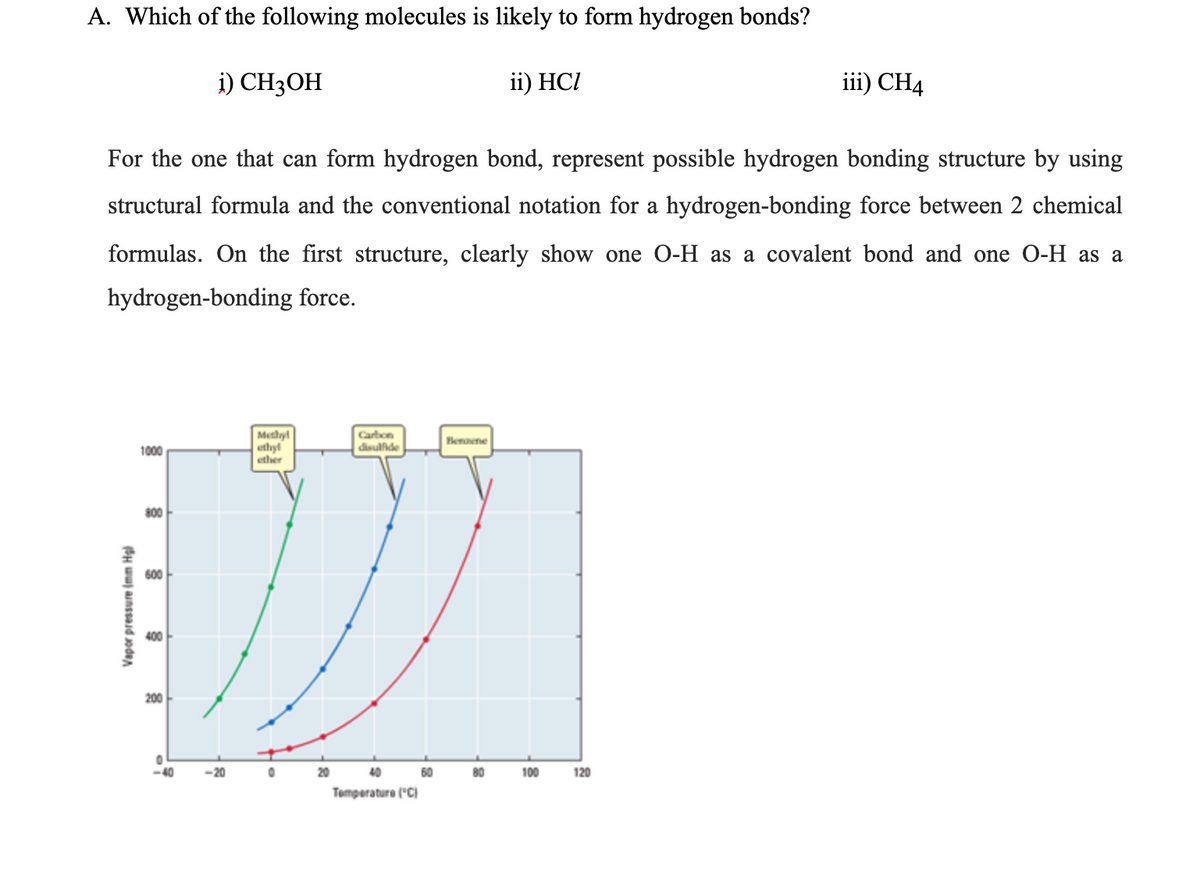
Answered A. Which of the following molecules is… bartleby
Web can ch3oh form a hydrogen bond? It cannot form hydrogen bonds with other ch₃br molecules. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. One consequence of this is that, even though dimethyl ether is a gas at room temperature, you can prepare a. There.
Solved 3. Which of the following molecules are capable of
3.om c6h1206 1.om cacl2 1.25m nacl 1.25m na2so4. It can form hydrogen bonds with other ch₃oh molecules. Web can ch3oh form a hydrogen bond? Hydrogen bonds are not as stable as covalences. Two with the hydrogen atoms and two with the with the oxygen atoms.
12.6 Intermolecular Forces Dispersion, DipoleDipole, Hydrogen
It can form hydrogen bonds with other ch₃oh molecules. Ch3ch2ch3 ch4 ch3coch3 ch3oh which salt would lower the freezing point of a solvent the most? 3.om c6h1206 1.om cacl2 1.25m nacl 1.25m na2so4. One consequence of this is that, even though dimethyl ether is a gas at room temperature, you can prepare a. Web in the case of ch₃oh, this.
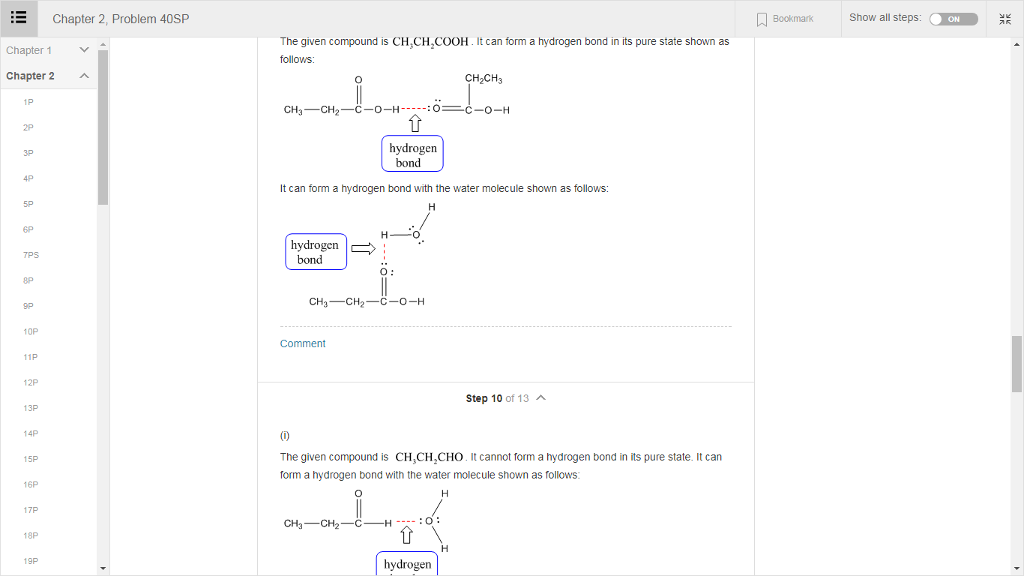
Solved Chapter 2, Problem 40SP Bookmark Show all steps The
Web in the case of ch₃oh, this molecule satisfies both the above conditions, that is, there is an electronegative oxygen atom and a directly attached hydrogen atom. Each doublet can attract one h atom from foreign molecules. Again ch3 + carbocation is very unstable. Web answer (1 of 5): Web notice that each water molecule can potentially form four hydrogen.
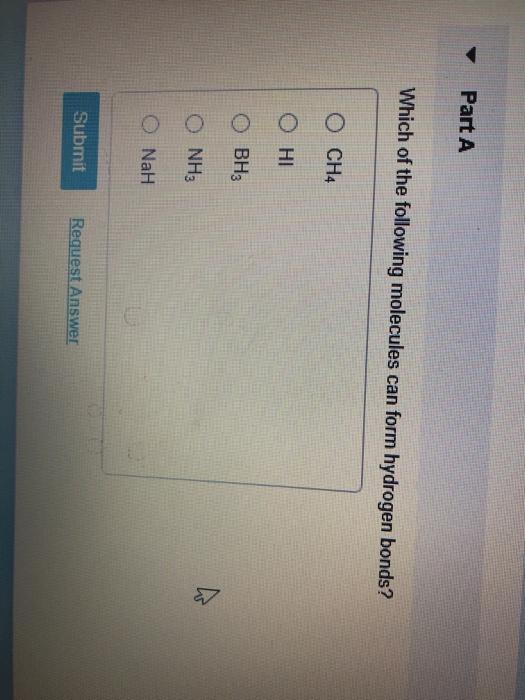
Solved Part A Which of the following molecules can form
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web $\begingroup$ i don't know whether this can be proven or disproven. Again ch3 + carbocation is very unstable. Web answer (1 of 5): In principle, the oxygen atom of ch3oh has two unused doublets.
PPT States of Matter PowerPoint Presentation ID542353
Web answer (1 of 10): There are exactly the right numbers of δ + hydrogens and lone pairs for every one of them to be involved in hydrogen bonding. It can form hydrogen bonds with other ch₃oh molecules. Web $\begingroup$ i don't know whether this can be proven or disproven. One consequence of this is that, even though dimethyl ether.
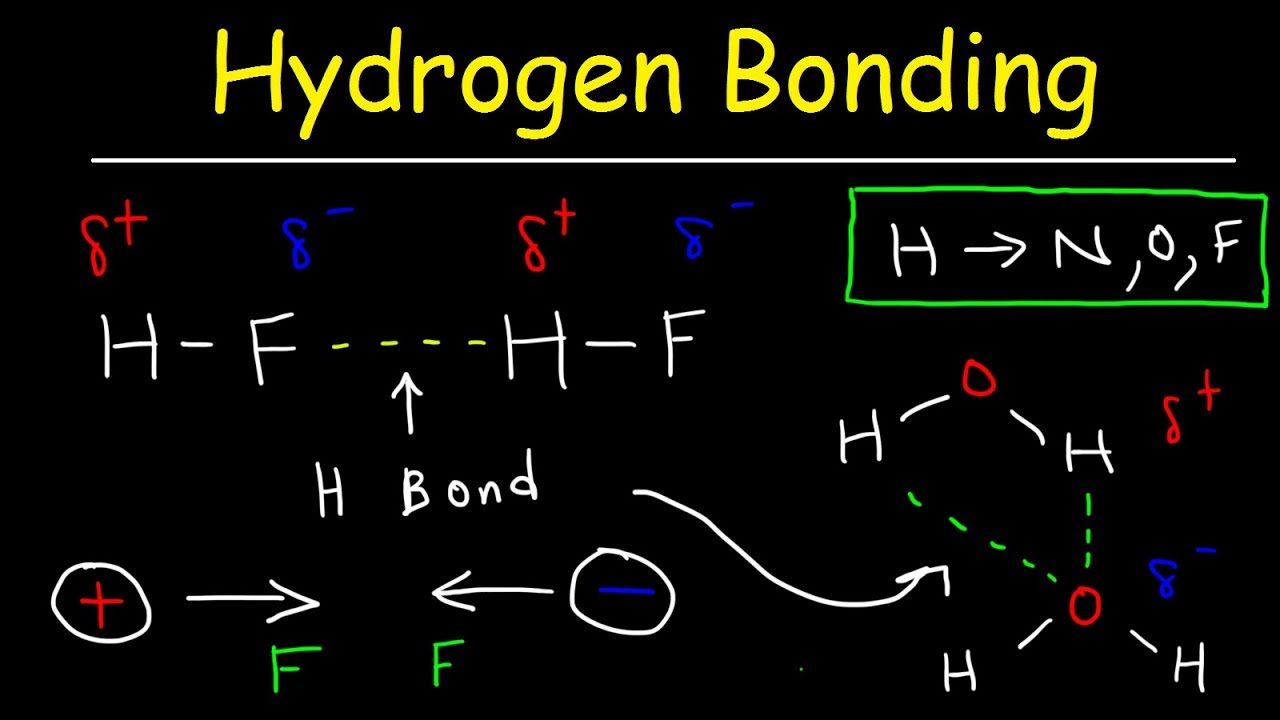
Web Hydrogen Bonds Can Form Between Different Molecules, As Long As One Molecule Has H And The Other Has N, O, Or F.
Ch₃br has no n, o, or f atoms, and it has no h atoms attached to n, o, or f. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web answer (1 of 5): It cannot form hydrogen bonds with other ch₃br molecules.
Which Of The Following Molecules Can Form Hydrogen Bonds In Samples Of The Pure Substance?
It can form hydrogen bonds with other ch₃oh molecules. Web answer (1 of 10): Ch3ch2ch3 ch4 ch3coch3 ch3oh which salt would lower the freezing point of a solvent the most? 3.om c6h1206 1.om cacl2 1.25m nacl 1.25m na2so4.
Two With The Hydrogen Atoms And Two With The With The Oxygen Atoms.
Web science chemistry chemistry questions and answers which molecule or molecules can form hydrogen bonds with water? In principle, the oxygen atom of ch3oh has two unused doublets. Web in the case of ch₃oh, this molecule satisfies both the above conditions, that is, there is an electronegative oxygen atom and a directly attached hydrogen atom. One consequence of this is that, even though dimethyl ether is a gas at room temperature, you can prepare a.
Hydrogen Bonds Are Not As Stable As Covalences.
Web can ch3oh form a hydrogen bond? So yes, we can have hydrogen bonding between one h2o molecule and one hcl molecule, in which case the o molecule in h2o forms a hydrogen bond with the h from hcl. Each doublet can attract one h atom from foreign molecules. Which molecule or molecules can form hydrogen bonds with water?