Crf Full Form In Clinical Research
Crf Full Form In Clinical Research - Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!). 2 meanings of crf abbreviation related to research: Web crf is a commonly used acronym for case report form; Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’. Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. Web case report form (crf) is a key document for data collection in clinical trials. Common terminology criteria for adverse events. Web what is crf meaning in research? For a study to be successful, the data collected must be correct and complete. Web a case report form (crf) is designed to collect the patient data in a clinical trial;
Case report form (crf) is a specialized document in clinical research. Its development represents a significant part of the clinical trial and can affect study success. Common terminology criteria for adverse events. Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!). Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’. A form which is used for capturing data in pharmaceutical and medical device clinical trials. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Web what is crf meaning in research? Web a case report form (crf) is designed to collect the patient data in a clinical trial; This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced.
Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced. It should be study protocol driven, robust in content and have material to collect the study specific data. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Web crf is a commonly used acronym for case report form; Web case report form (crf) refers to a form that collects patient data that investigators use during a visit. Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’. A form which is used for capturing data in pharmaceutical and medical device clinical trials. Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. Common terminology criteria for adverse events.
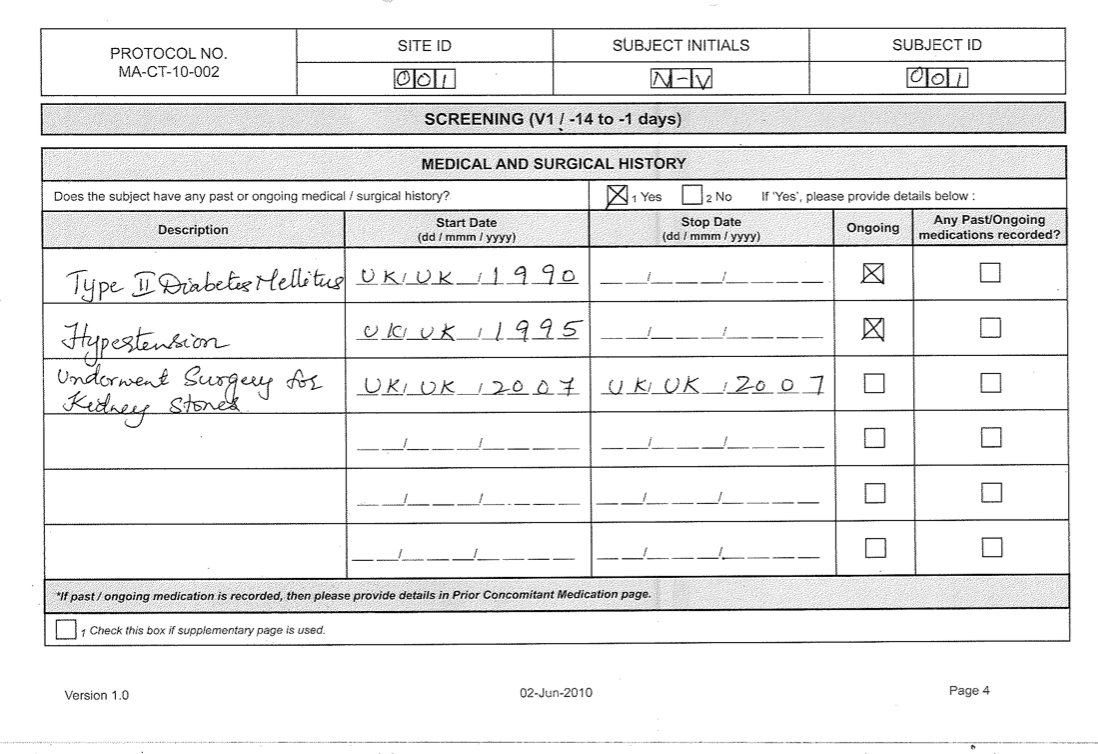
Case Report Form (CRF) for clinical examination 1. CRF to be filled by
Web case report form (crf) is a key document for data collection in clinical trials. Its development represents a significant part of the clinical trial and can affect study success. Electronic crfs are powered by automation technology. A form which is used for capturing data in pharmaceutical and medical device clinical trials. This data can include everything from participants’ personal.
Usoda Monumentalno analogija case report form poglej izgovorjava Opis
A form which is used for capturing data in pharmaceutical and medical device clinical trials. Case report form (crf) is a specialized document in clinical research. Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’. Case report forms (crfs) have historically always been on something like carbon copy paper, so.
CRF Full Form In Hindi CRF Se Kya Samjhte Hai PagalNews
Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!). Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. Electronic crfs are.
Query Management in Field Research (Beginner's Guide)
Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. Electronic crfs are powered by automation technology. Common terminology criteria for adverse events. 2 meanings of crf abbreviation related to research: Case report form (crf) is a specialized document in clinical research.
Form E Report E2 80 93 Riat Support Center Crf Templates Intended For
This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced. Web crf is a commonly used acronym for case report form; Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. Case report form (crf) is a specialized document in clinical research. 2 meanings.
Full form of CRF Relejuvant Clinical Services
Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!). Web what is crf meaning in research? Web case report form (crf) is a key document for data collection in clinical trials. It should be study protocol driven,.
Longterm followup form. Simple one page CRF for collection of primary
Electronic crfs are powered by automation technology. Web case report form (crf) refers to a form that collects patient data that investigators use during a visit. Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. Common terminology criteria for adverse events. Though paper crfs are still used largely, use of electronic crfs (ecrfs).
PPT SAFETY MONITORING IN CLINICAL TRIALS PowerPoint Presentation
Web case report form (crf) is a key document for data collection in clinical trials. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced. Web what is crf.
Form Crf004 Additional Ownership / Relationship Form printable pdf
Web case report form (crf) refers to a form that collects patient data that investigators use during a visit. Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. This data can include everything from participants’ personal information to study visit results to treatment outcomes.
Usoda Monumentalno analogija case report form poglej izgovorjava Opis
A form which is used for capturing data in pharmaceutical and medical device clinical trials. Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. It should be study protocol driven, robust in content and have material to collect the study specific data. Web a case report form (crf) is designed to collect the.
Crfs Play A Crucial Role In Helping To Assess The Safety And Efficacy Of Clinical Products.
Web case report form (crf) refers to a form that collects patient data that investigators use during a visit. This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced. It should be study protocol driven, robust in content and have material to collect the study specific data. Common terminology criteria for adverse events.
Case Report Form (Crf) Is A Specialized Document In Clinical Research.
Web crf is a commonly used acronym for case report form; For a study to be successful, the data collected must be correct and complete. Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!). Its development represents a significant part of the clinical trial and can affect study success.
Web Case Report Form (Crf) Is A Key Document For Data Collection In Clinical Trials.
Web a case report form (crf) is designed to collect the patient data in a clinical trial; Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’. Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. Electronic crfs are powered by automation technology.
[ 1] Site Personnel Capture The Subject's Data On The Crf, Which Is Collected During Their Participation In A Clinical Trial.
A form which is used for capturing data in pharmaceutical and medical device clinical trials. 2 meanings of crf abbreviation related to research: Web what is crf meaning in research?