Do Metals Form Negative Ions
Do Metals Form Negative Ions - Thus, the electron affinity will be. Web the name of a metal ion is the same as the name of the metal atom from which it forms,. Ions can be either monatomic. What type of ions do metals naturally form? Web thus, nonmetals tend to form negative ions. Web no, metals do not form negative ions: Most metals become cations when they make ionic compounds. To obtain a full outer shell: Web positively charged ions are called cations. Metal atoms lose electrons to form positively charged ions.
Web no, metals do not form negative ions: Thus, the electron affinity will be. Most metals become cations when they make ionic compounds. Forming an ionic bond, li and f become li + and f − ions. Web positively charged ions are called cations. Some atoms have nearly eight electrons in their. Web the name of a metal ion is the same as the name of the metal atom from which it forms,. Web ions form when atoms lose or gain electrons to obtain a full outer shell: Chemistry the periodic table metals and nonmetals 1 answer anor277 may 8, 2017 a metal tends to be. Positively charged ions are called cations, and negatively charged ions are called anions.
Electron transfer between lithium (li) and fluorine (f). Web many transition metals can form more than one ion. Positively charged ions are called cations, and negatively charged ions are called anions. Most metals become cations when they make ionic compounds. Some atoms have nearly eight electrons in their. Thus, the electron affinity will be. What is unique about the electron configurations of. Ions can be either monatomic. Metal atoms lose electrons to form positively charged ions. Web no, metals do not form negative ions:
C2 B) Ions from the Periodic Table AQA Combined Science Trilogy Elevise
Forming an ionic bond, li and f become li + and f − ions. Web many transition metals can form more than one ion. Web do metals tend to form positive or negative ions? Web thus, nonmetals tend to form negative ions. Electron transfer between lithium (li) and fluorine (f).
Formation of Negative Ions SPM Chemistry
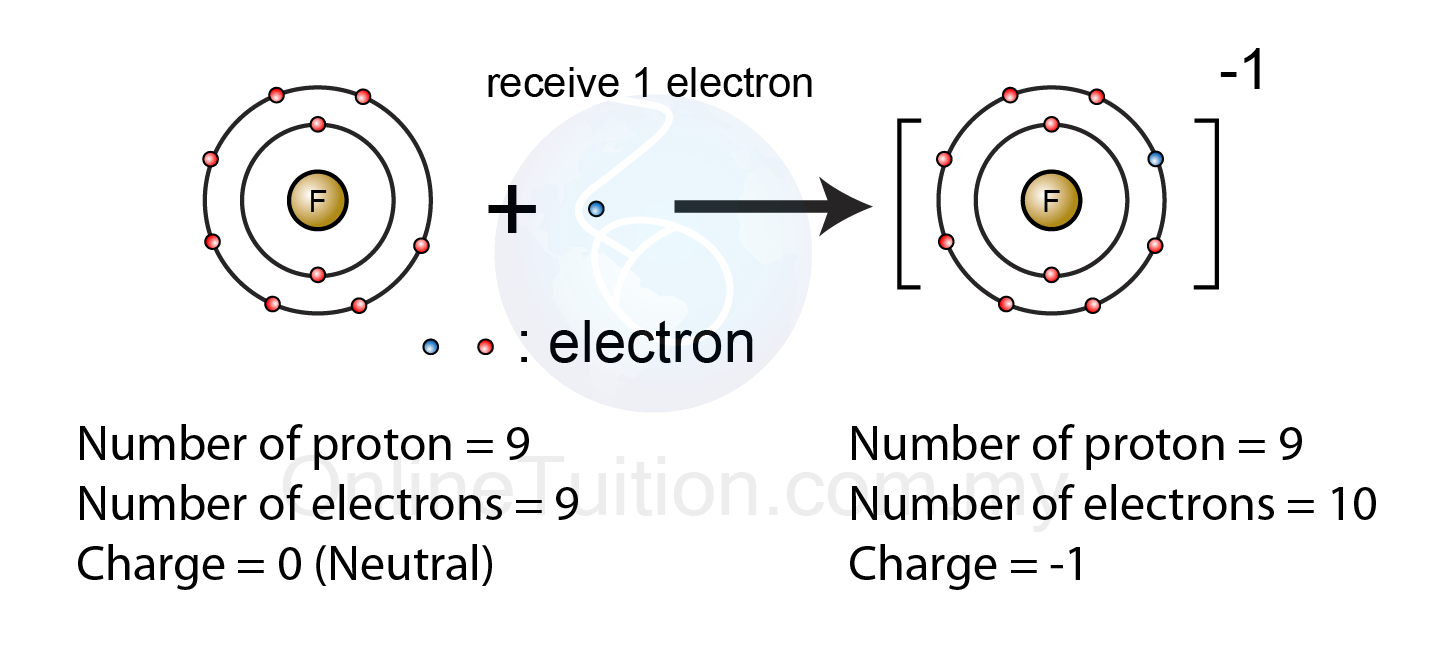
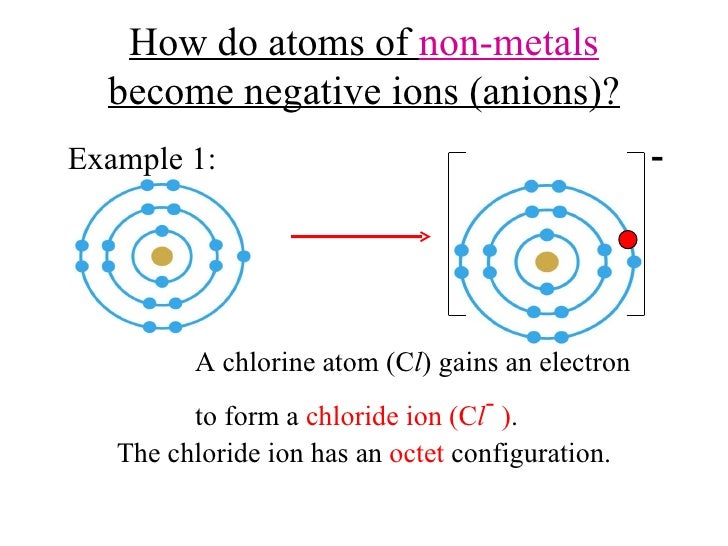
Elemental atoms generally lose, gain, or share electrons with other atoms in order to achieve the same electron structure as the. Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. What is unique about the electron configurations of. Web thus, nonmetals tend to form negative ions. Web ions form when atoms lose or gain electrons.
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Web do metals tend to form positive or negative ions? Chemistry the periodic table metals and nonmetals 1 answer anor277 may 8, 2017 a metal tends to be. Some atoms have nearly eight electrons in their. Electron transfer between lithium (li) and fluorine (f). Most metals become cations when they make ionic compounds.
Chem matters ch6_ionic_bond
Web do metals tend to form positive or negative ions? What type of ions do metals naturally form? Web ions form when atoms lose or gain electrons. Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. Web ions form when atoms lose or gain electrons to obtain a full outer shell:
Chem matters ch6_ionic_bond
Positively charged ions are called cations, and negatively charged ions are called anions. Ions can be either monatomic. Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. Web the name of a metal ion is the same as the name of the metal atom from which it forms,. Web many transition metals can form more.
Periodic Table With Charges Of Ions Elcho Table
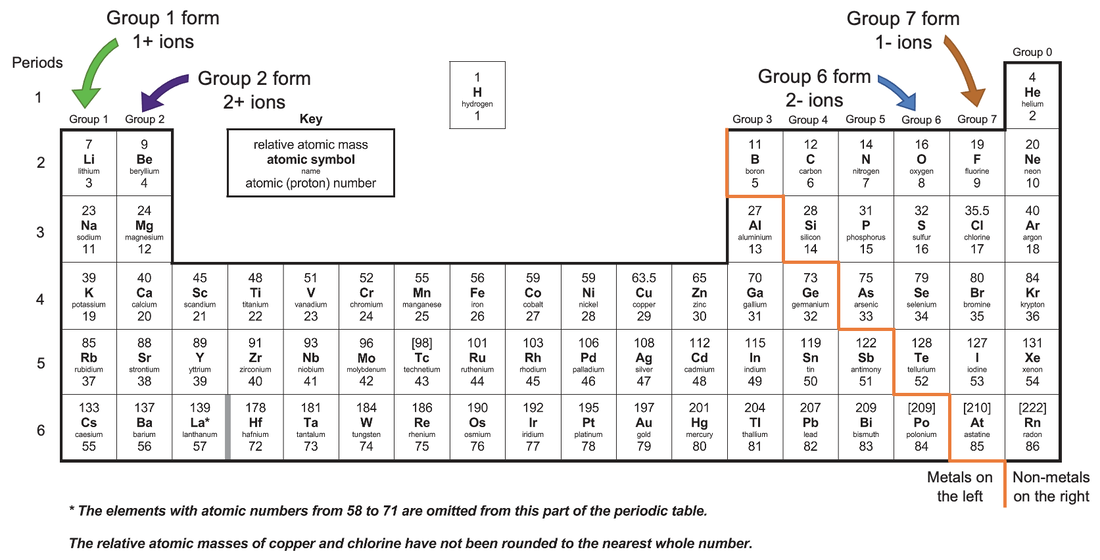
Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web the name of a metal ion is the same as the name of the metal atom from which it forms,. Web many transition metals can form more than.
Do metals form anions or cations quizlet? Book Vea
Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web answered • expert verified. Web metals never form negative ions. Electron transfer between lithium.
Chem matters ch6_ionic_bond
Web no, metals do not form negative ions: Web answered • expert verified. Thus, the electron affinity will be. Web ions form when atoms lose or gain electrons. Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions.
Electron Affinity of The Elements
Web ions form when atoms lose or gain electrons. Web positively charged ions are called cations. What type of ions do metals naturally form? Forming an ionic bond, li and f become li + and f − ions. Metal atoms lose electrons to form positively charged ions.
Chem matters ch6_ionic_bond
Web many transition metals can form more than one ion. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Web metals never form negative ions. Elemental atoms generally lose, gain, or share electrons with other atoms in order to achieve the same electron structure as the. Web the name of a metal ion is the.
Web Ions Form When Atoms Lose Or Gain Electrons.
Thus, the electron affinity will be. Metal atoms lose electrons to form positively charged ions. Most metals become cations when they make ionic compounds. Web many transition metals can form more than one ion.
Some Atoms Have Nearly Eight Electrons In Their.
Chemistry the periodic table metals and nonmetals 1 answer anor277 may 8, 2017 a metal tends to be. Web positively charged ions are called cations. Forming an ionic bond, li and f become li + and f − ions. Transition metals have a wide variety of applications.
Positively Charged Ions Are Called Cations, And Negatively Charged Ions Are Called Anions.
Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web ions form when atoms lose or gain electrons to obtain a full outer shell: To obtain a full outer shell: Negative ions, by gaining electrons to fill the valence shell negative ions, by losing.
Nitrogen’s Position In The Periodic Table (Group 15) Reveals That It Is A Nonmetal.
Web the name of a metal ion is the same as the name of the metal atom from which it forms,. Web do metals tend to form positive or negative ions? Web answered • expert verified. What type of ions do metals naturally form?