Does Sulfur And Iodine Form An Ionic Compound
Does Sulfur And Iodine Form An Ionic Compound - Web what does calcium and sulfur make? Predict the type of compound formed from elements based on their location within the periodic table. The electronegativities of sulfur and iodine are 2.58 and 2.66 respectively so we would predict that they would form a covalent bond. Web if they will, write the empirical formula of the compound formed in the space provided. Web molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the. Web define ionic and molecular (covalent) compounds. What is the charge on its ions, and is the charge positive. Iodine is in group 7. An alternative way to writing a correct formula for an ionic compound is to. Calcium sulfide calcium and sulfur form calcium sulfide.
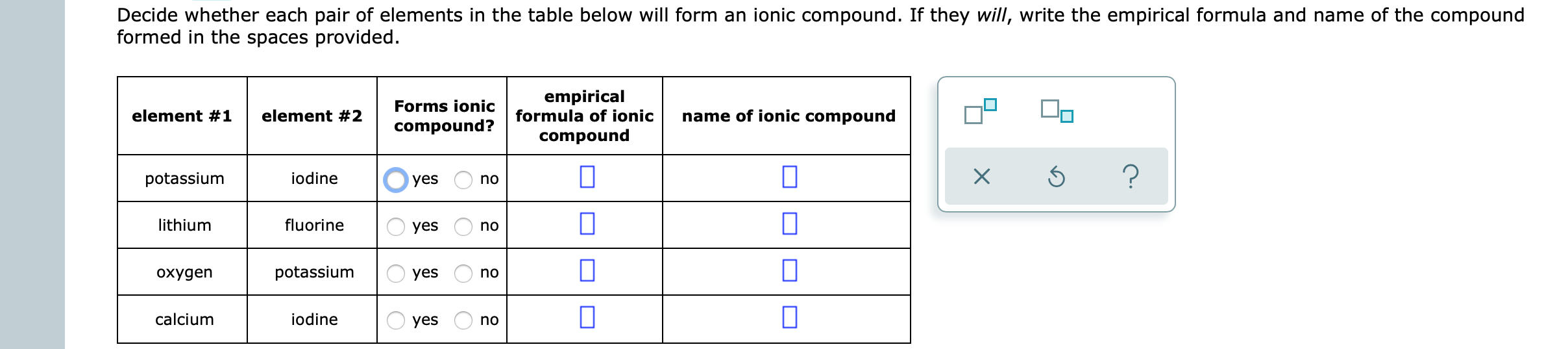
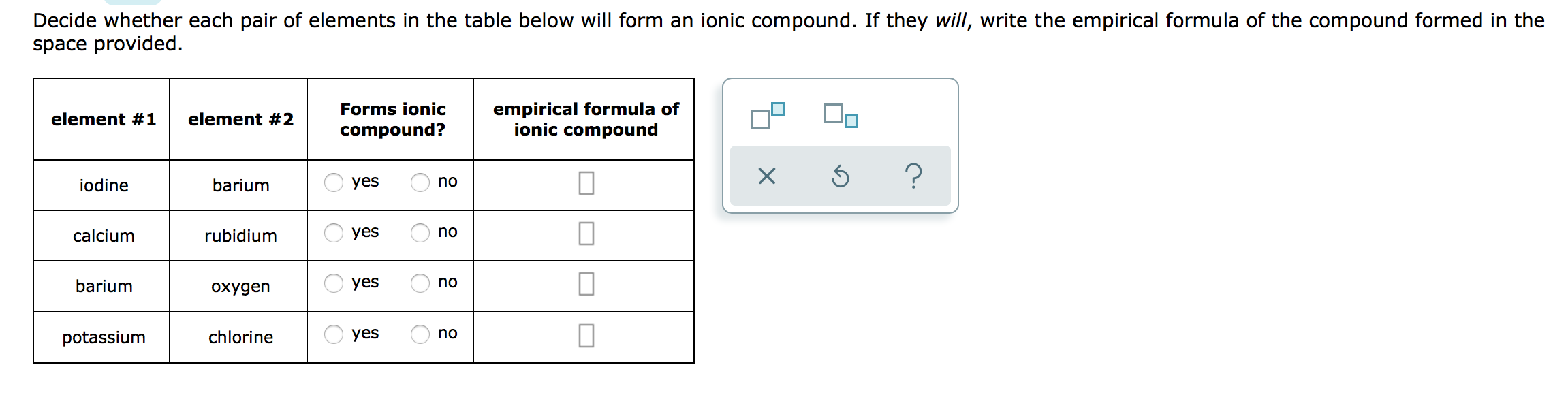
Sulfur diiodide (si 2) has finally been reported in an argon matrix at 9 k by the reaction of sulfur dichloride and iodine; Web determine the chemical formula for the ionic compound that forms between cesium and iodine. If you mix sulphuric acid with iodine (i2), not much. Iodine is in group 7. An alternative way to writing a correct formula for an ionic compound is to. Determine the chemical formula for the ionic compound that. Probably dissolve the iodine so the mess will become somewhat purple in color. Ionic compound formed from aluminum and fluorine; Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п bromine sodium yes no 0 lithium sulfur. However, this has been disputed.
The electronegativities of sulfur and iodine are 2.58 and 2.66 respectively so we would predict that they would form a covalent bond. However, this has been disputed. If you mix sulphuric acid with iodine (i2), not much. Iodine can form compounds using multiple oxidation states. Web molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the. Now, if you meant to ask what will. Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п bromine sodium yes no 0 lithium sulfur. Iodine is in group 7. Determine the chemical formula for the ionic compound that. Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of purity—colorless gases at room temperature.
Answered Decide whether each pair of elements in… bartleby
An alternative way to writing a correct formula for an ionic compound is to. Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of purity—colorless gases at room temperature. The electronegativities of sulfur and iodine are 2.58 and 2.66 respectively so we would predict that they would form a covalent bond. Web.
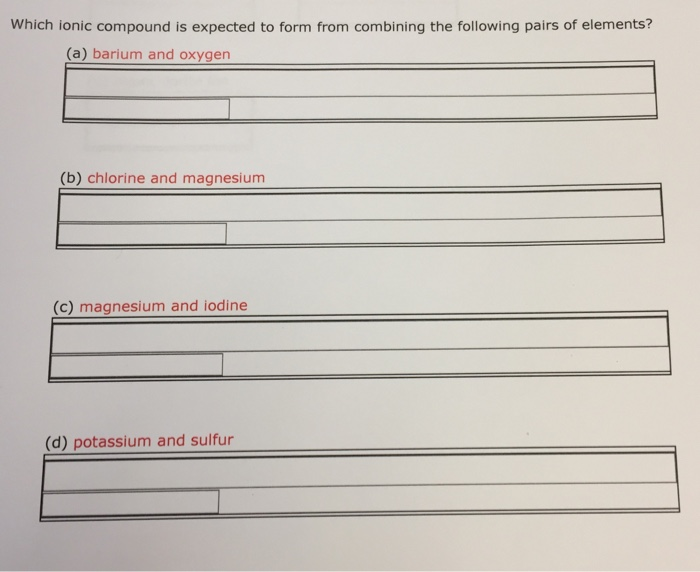
Solved Which ionic compound is expected to form from
Now, if you meant to ask what will. Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п bromine sodium yes no 0 lithium sulfur. Solution verified create an account to view solutions An alternative way to writing a correct formula for an ionic compound is to. Sulfur diiodide (si 2) has finally been reported in.
Solved How many valence electrons are in the compound
An alternative way to writing a correct formula for an ionic compound is to. Predict the type of compound formed from elements based on their location within the periodic table. Ionic compound formed from aluminum and fluorine; Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п bromine sodium yes no 0 lithium sulfur. Sulfur diiodide.
does barium and rubidium form an ionic compound (2023)
Web element #1 element #2 forms ionic compound? Now, if you meant to ask what will. Empirical formula of ionic compound. Leave out all charges and all subscripts that are 1. Iodine can form compounds using multiple oxidation states.
Ionic Properties
Ionic compound formed from iron and oxygen (assume the iron ion takes. Ionic compound formed from aluminum and fluorine; Leave out all charges and all subscripts that are 1. If you mix sulphuric acid with iodine (i2), not much. Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient levels of purity—colorless gases at.
Which Of These Pairs Of Elements Would Be Most Lik...
Predict the type of compound formed from elements based on their location within the periodic table. Calcium sulfide calcium and sulfur form calcium sulfide. Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п bromine sodium yes no 0 lithium sulfur. Web define ionic and molecular (covalent) compounds. Does lithium and iodine form an ionic compound?
Crystals of Iodine, Iodine in Metal Form Stock Image Image of
Now, if you meant to ask what will. What is the charge on its ions, and is the charge positive. Web define ionic and molecular (covalent) compounds. Calcium sulfide calcium and sulfur form calcium sulfide. Predict the type of compound formed from elements based on their location within the periodic table.
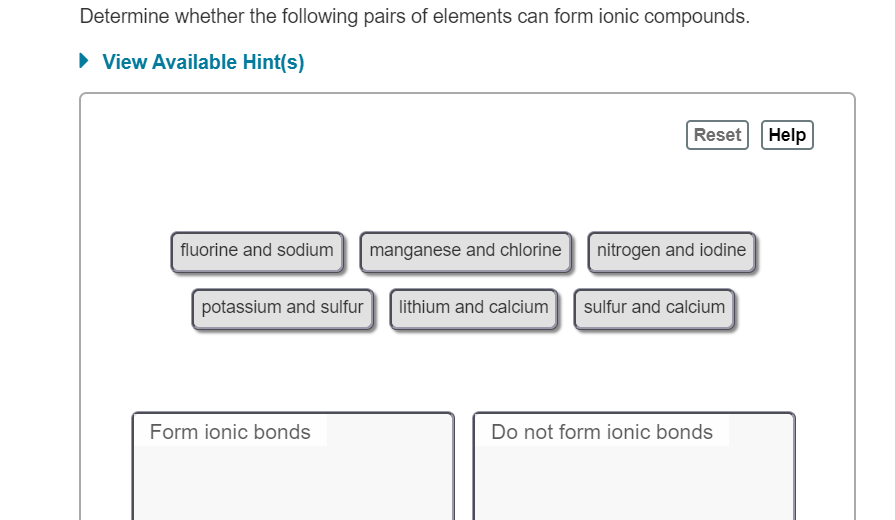
Solved Determine Whether the following pairs of elements can
Element #1 element #2 forms ionic compound? Web ionic compound formed from sodium and sulfur; However, this has been disputed. Determine the chemical formula for the ionic compound that. Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п bromine sodium yes no 0 lithium sulfur.
Solved What is the formula for an ionic compound that
Now, if you meant to ask what will. What is the charge on its ions, and is the charge positive. Probably dissolve the iodine so the mess will become somewhat purple in color. Iodine is in group 7. An alternative way to writing a correct formula for an ionic compound is to.
Solved Decide whether each pair of elements in the table
Leave out all charges and all subscripts that are 1. What is the charge on its ions, and is the charge positive. Iodine is in group 7. Web what does calcium and sulfur make? Web molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in.
Empirical Formula Of Ionic Compound Iodine Sulfur Yes No Chlorine Calcium Yes No П Bromine Sodium Yes No 0 Lithium Sulfur.
Calcium sulfide calcium and sulfur form calcium sulfide. Iodine is quite reactive, but it is much less reactive than the other halogens. If you mix sulphuric acid with iodine (i2), not much. Web determine the chemical formula for the ionic compound that forms between cesium and iodine.
Ionic Compound Formed From Iron And Oxygen (Assume The Iron Ion Takes.
Iodine is in group 7. The electronegativities of sulfur and iodine are 2.58 and 2.66 respectively so we would predict that they would form a covalent bond. Ionic compound formed from aluminum and fluorine; What is the charge on its ions, and is the charge positive.
However, This Has Been Disputed.
Empirical formula of ionic compound. Web what does calcium and sulfur make? Iodine is in group 7. Web ionic compound formed from sodium and sulfur;
Solution Verified Create An Account To View Solutions
Leave out all charges and all subscripts that are 1. Determine the chemical formula for the ionic compound that forms between lithium and iodine. Sulfur diiodide (si 2) has finally been reported in an argon matrix at 9 k by the reaction of sulfur dichloride and iodine; Web if they will, write the empirical formula of the compound formed in the space provided.