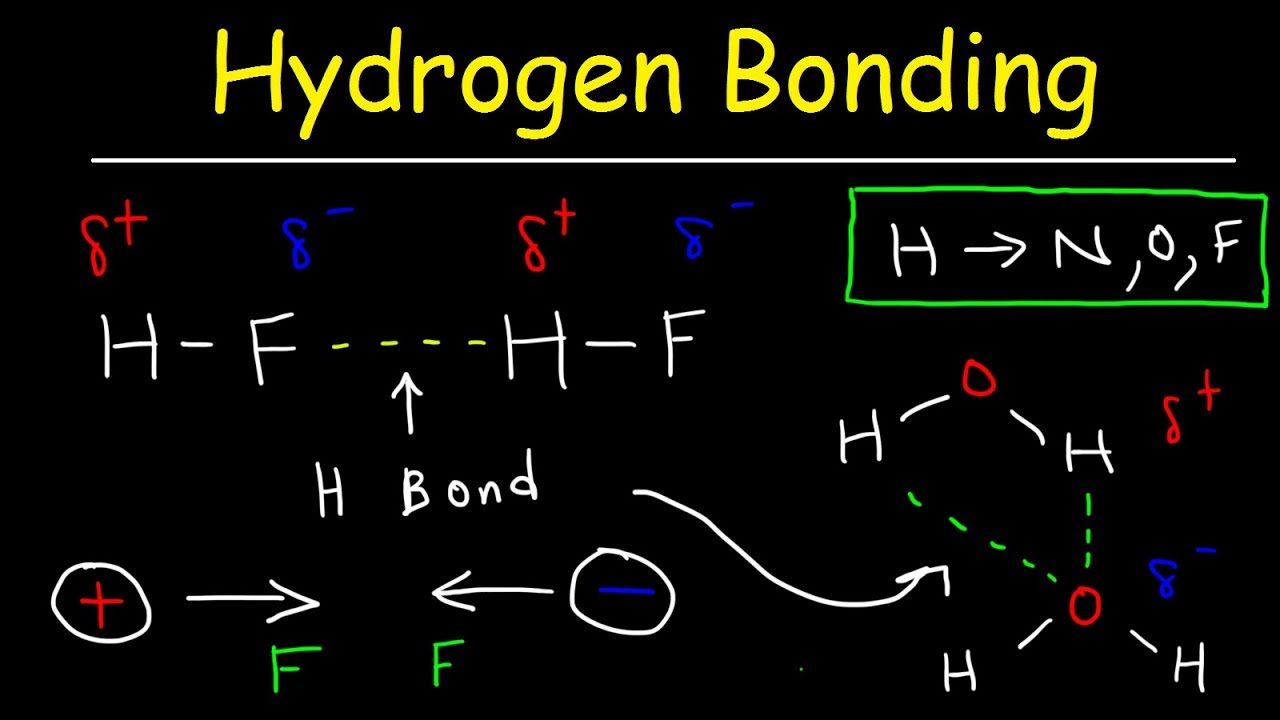
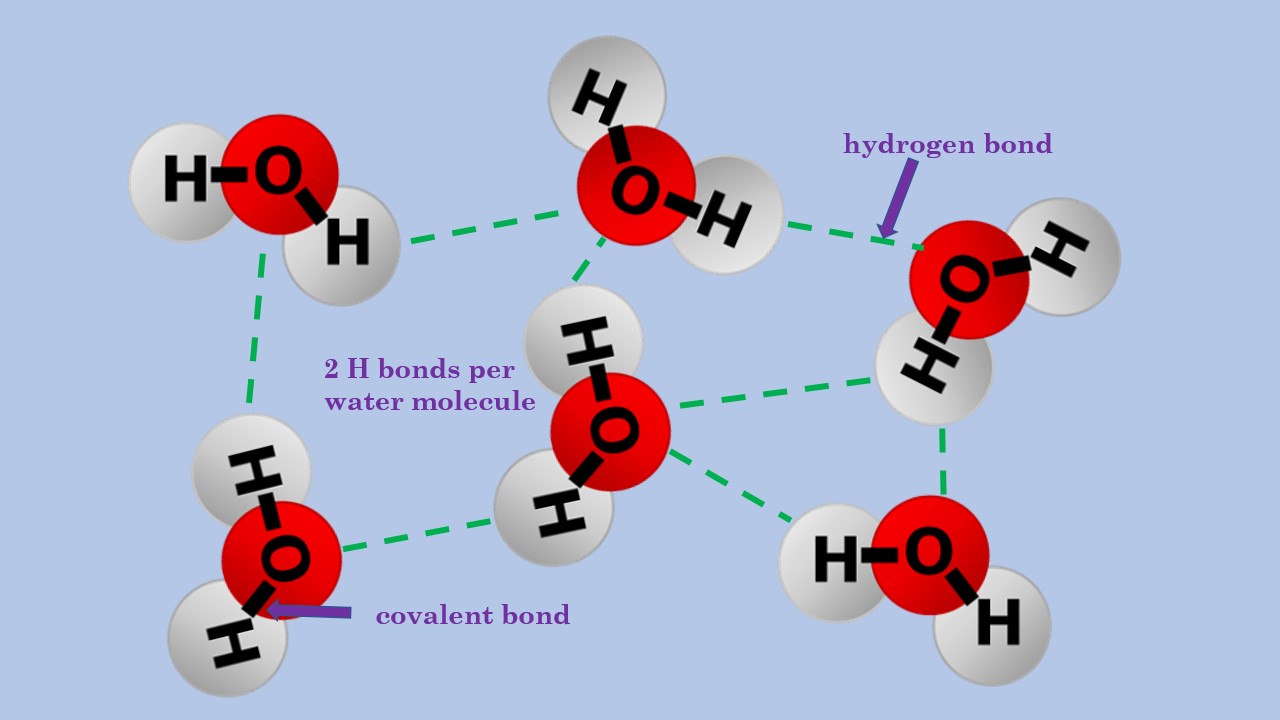
Draw A Hydrogen Bond Between Two Water Molecules
Draw A Hydrogen Bond Between Two Water Molecules - Draw the hydrogen bonds that could form between water molecules and the appropriaate eregions draw the hydrogen bonds that could form between water molecules and the appropriaate eregi there’s just one step to solve this. Use dashed lines ( ) to represent the hydrogen bond, not a solid line (which represents a covalent bond). Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. (3 points) draw a hydrogen bond between two water molecules. B) clearly name and label the type of bond that exists between the oxygen and hydrogen atoms within one of the molecules. A) draw two water molecules. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. (1 point) what type of bond involves the sharing of electrons between two atoms? Web there are two requirements for hydrogen bonding.
Study the water molecules at the right. (hydrogen bond donor) second molecule has a lone pair of electrons on a small highly electronegative atom (n,o,f). Web each water molecule can form a total of 4 hydrogen bonds. However, because they are exposed to air on one side, they will have fewer neighboring water molecules to bond with, and will form stronger bonds with the neighbors they do have. This is why the boiling point of water is higher than that of ammonia or hydrogen fluoride. So, if you are looking to draw a hydrogen bond between two molecules, look for two things. There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. C) clearly name and label the type of bond that exists between the oxygen and hydrogen atoms of the two separate water molecules. Draw the hydrogen bonds that are possible between water molecules and an ethanol molecule. A) draw two water molecules.
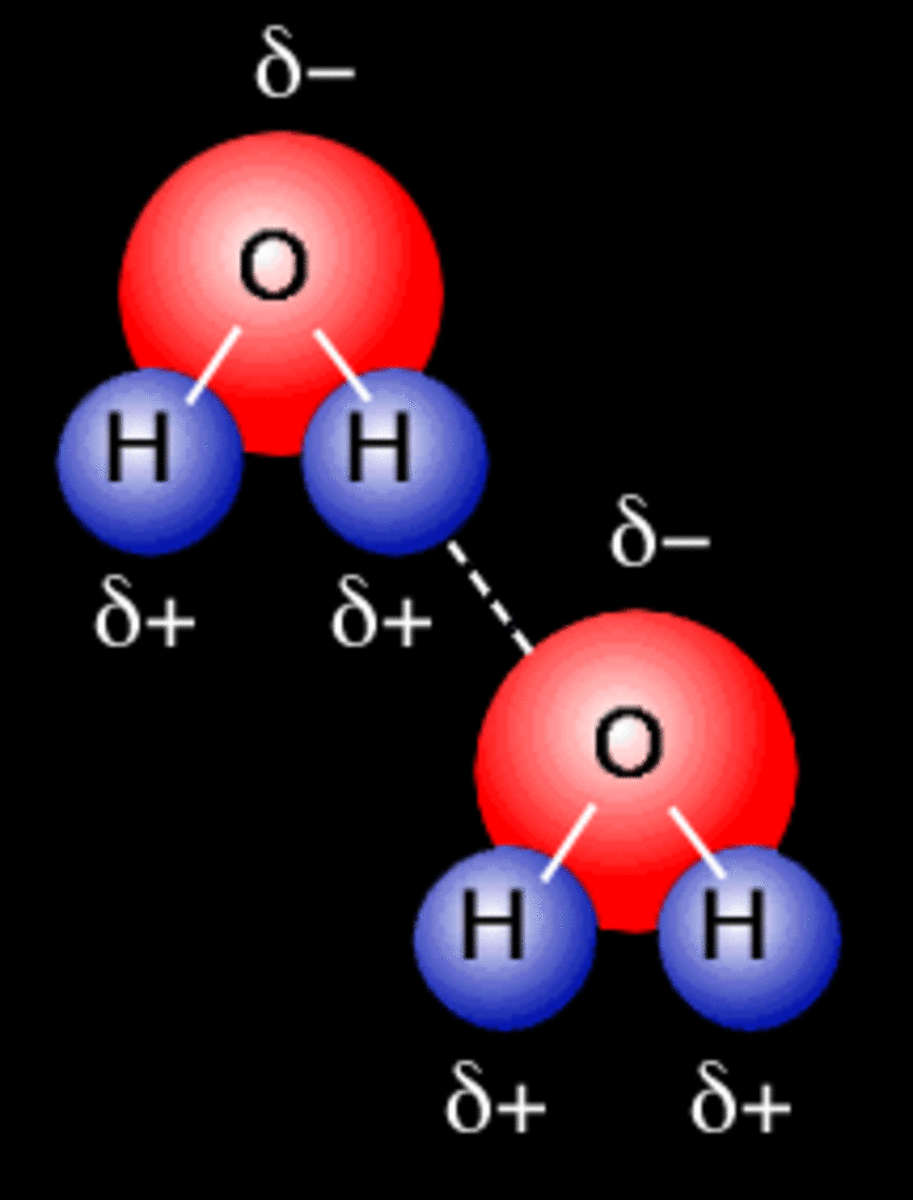
Hydrogen atoms attached to fluorine, oxygen, or nitrogen on one molecule; Be sure to clearly represent the important aspects of the structure of each water molecule in your drawing. Web the hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. (hydrogen bond donor) second molecule has a lone pair of electrons on a small highly electronegative atom (n,o,f). Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent (polar bonds). Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Besides hydrogen bonds, what other intermolecular forces could be possible between a water molecule and an ethanol molecule? Draw the hydrogen bonds that are possible between water molecules and an ethanol molecule. Web this is why each water molecule can form hydrogen bonds to 4 other water molecules. Web biology document from seoul foreign school, 3 pages, name:
Hydrogen bond between two water molecules
There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. This is why the boiling point of water is higher than that of ammonia or hydrogen fluoride. B) clearly name and label the type of bond that exists between the oxygen and hydrogen atoms within one.
Diagram Of Water Molecules Hydrogen Bonding
There are exactly the right numbers of δ+ hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. Web water molecules forming hydrogen bonds with one another. First molecules has hydrogen attached to a highly electronegative atom (n,o,f). Ph and water 2.5 hydrogen bonding gives water properties that help make life possible on earth.
Science online The importance of the water and its structure
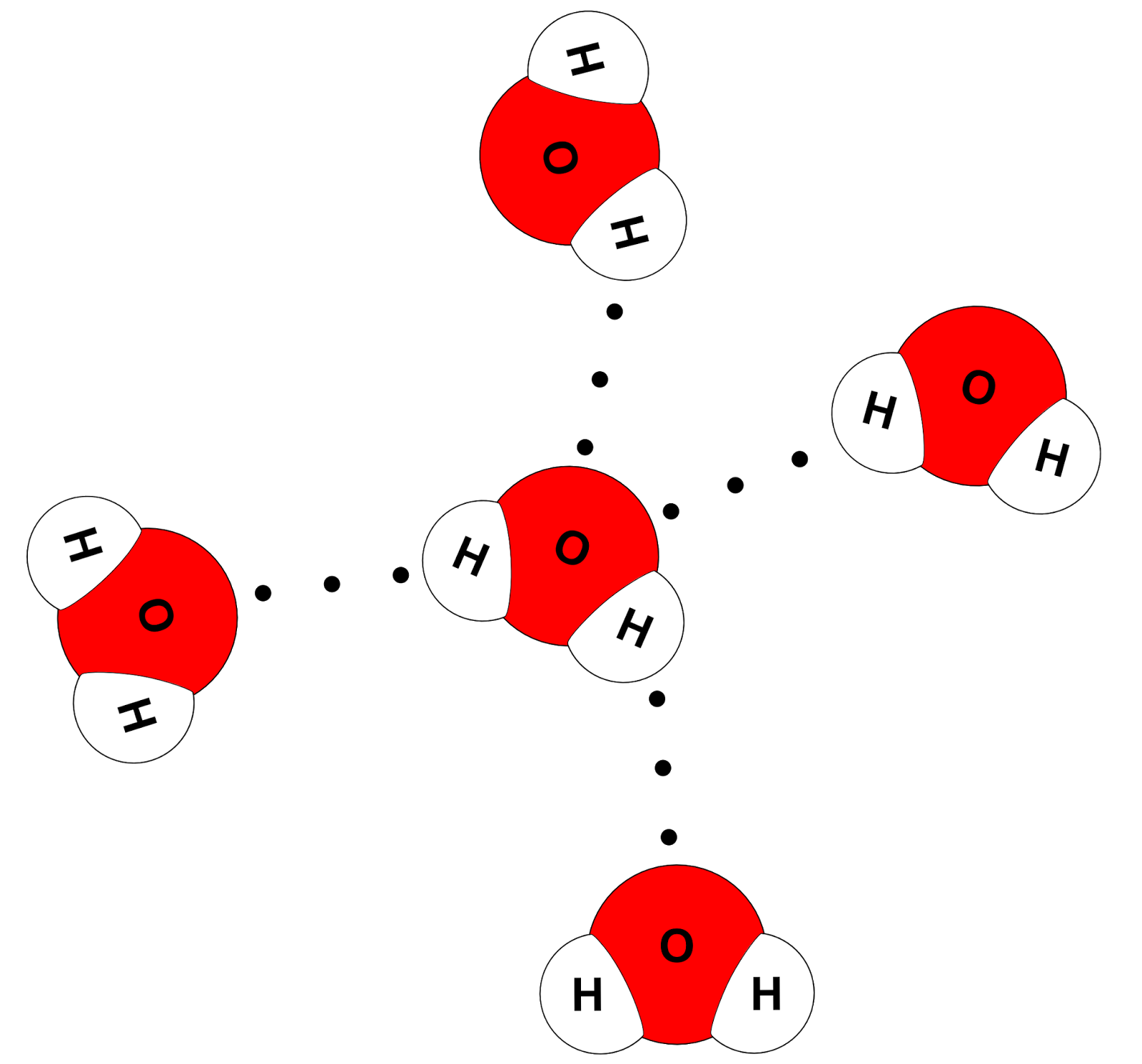
Two requirements for hydrogen bonding: Web each water molecule can form a total of 4 hydrogen bonds. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. There are two lone pairs of electrons. Lone pairs of electrons on another molecule.
Primary and Secondary Bonds Owlcation
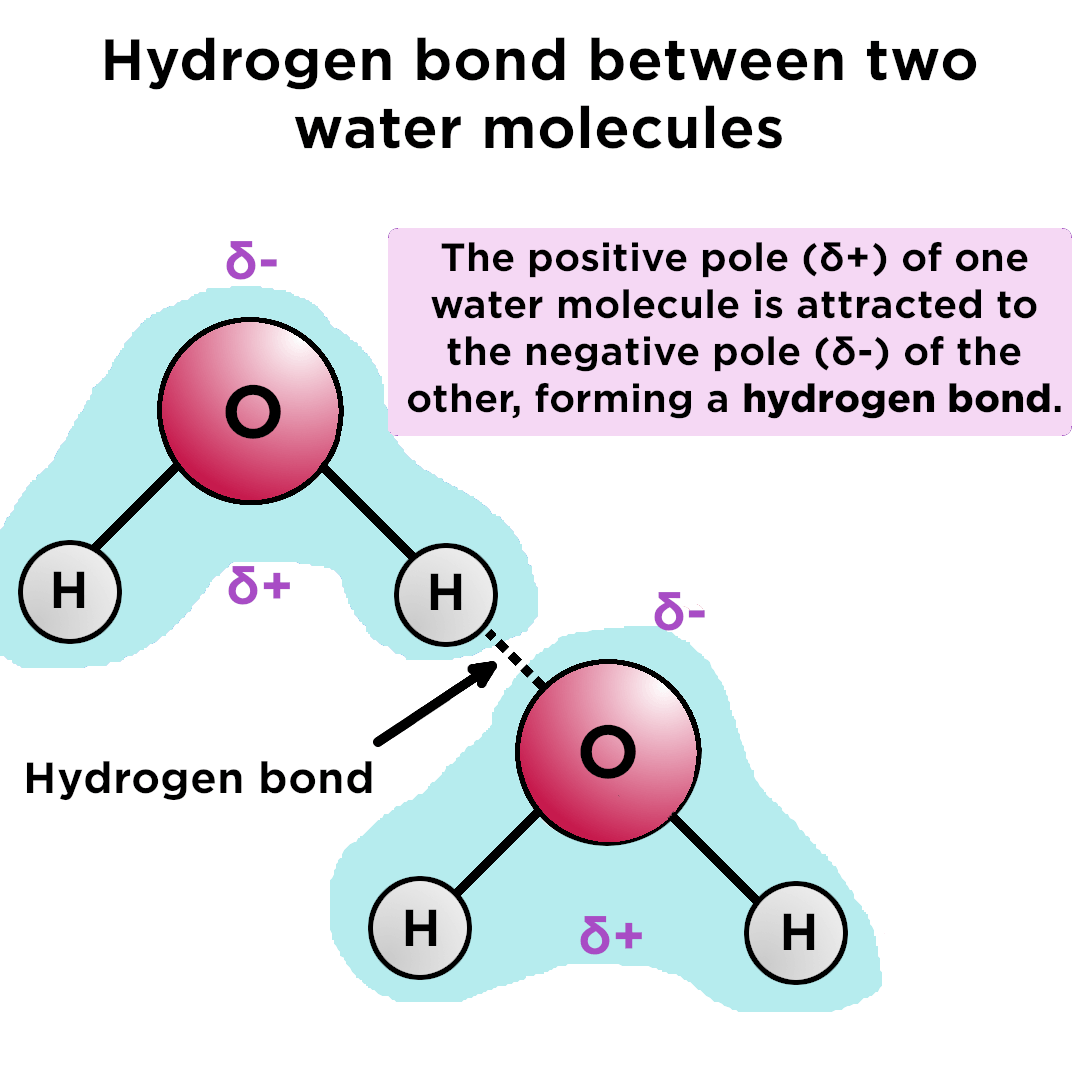
So, water molecules are able to form hydrogen bonds with one another, giving water many of its unique properties. Two requirements for hydrogen bonding: The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. Web hydrogen bonding between different parts of the same chain.
High Specific Heat (Water) — Properties & Examples Expii
Web water as a perfect example of hydrogen bonding. There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. (3 points) draw a hydrogen bond between two water molecules. This problem has been solved! This is why the boiling point of water is higher than that.
Draw a diagram of water molecules, labeling the hydrogen bond and
84k views 11 years ago. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch. The examples that follow are representative of several types of biopolymers. Ph and water 2.5 hydrogen bonding gives water properties that help make life possible on earth 1. Study the water molecules at the right.
Water has both a hydrogen bond and a polar covalent bond. Hydrogen
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. This is why the boiling point of water is higher. Two requirements for hydrogen bonding: Hydrogen bonding between different parts of the same chain (intramolecular bonding; Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules:
hydrogen bond between water molecules Diagram Quizlet
Lone pairs of electrons on another molecule. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent (polar bonds). Web water is an ideal example of hydrogen bonding. Web biology document from seoul foreign school, 3 pages, name: There are exactly the right numbers of δ+ hydrogens and lone pairs so that every one of them.
Hydrogen Bonding in water Dr. M. Chemistry Tutor
Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. On the central molecule, label oxygen (o) an Study the water molecules at the right. There are two lone pairs of electrons.
Diagram of hydrogen bonding between two water molecules Download
Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web water is an ideal example of hydrogen bonding. This is why the boiling point of water is higher than that of ammonia or hydrogen fluoride. Web coefficient means that there are two molecules of water. Notice that each water molecule can potentially.
First Molecules Has Hydrogen Attached To A Highly Electronegative Atom (N,O,F).
Draw the hydrogen bonds that could form between water molecules and the appropriaate eregions draw the hydrogen bonds that could form between water molecules and the appropriaate eregi there’s just one step to solve this. Web water molecules forming hydrogen bonds with one another. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch.
Notice That Each Water Molecule Can Potentially Form Four Hydrogen Bonds With Surrounding Water Molecules.
Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. 84k views 11 years ago. Web there are two requirements for hydrogen bonding. Since each water molecule has two hydrogen atoms and there are two water molecules, there must be 2 x 2 = 4 hydrogen atoms.
Be Sure To Clearly Represent The Important Aspects Of The Structure Of Each Water Molecule In Your Drawing.
Web water is an ideal example of hydrogen bonding. (hydrogen bond donor) second molecule has a lone pair of electrons on a small highly electronegative atom (n,o,f). Use dashed lines ( ) to represent the hydrogen bond, not a solid line (which represents a covalent bond). It is an electrostatic attraction between two polar groups.
(4 Pts) This Problem Has Been Solved!
So, if you are looking to draw a hydrogen bond between two molecules, look for two things. Web each water molecule can form a total of 4 hydrogen bonds. Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules.