Draw A Successive Ionization Energy Diagram For Aluminum
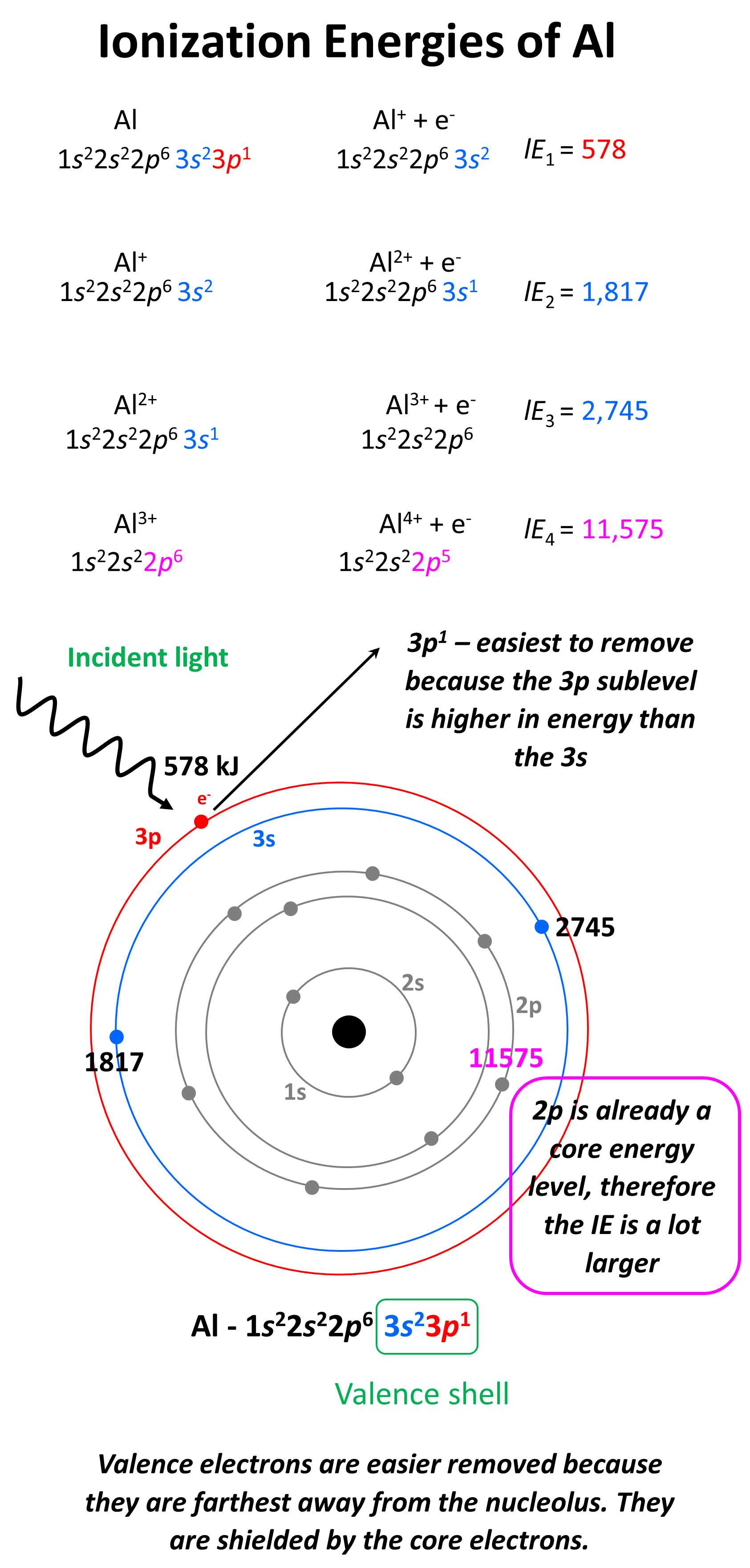
Draw A Successive Ionization Energy Diagram For Aluminum - Web the size of the first ionisation energy is affected by four factors: Shielding effect of inner electrons. Web you can then have as many successive ionisation energies as there are electrons in the original atom. Since these processes will both begin from a cationic state, the electrons will be more difficult to. Electron affinity and electronegativity of aluminum. For strong lines (both in atoms and in ions), it is of the order of unity. Web for example, sc and ga both have three valence electrons, so the rapid increase in ionization energy occurs after the third ionization. For aluminum, this is the 3p electron. Web the successive ionization energy diagram is shown in the picture below. 4th ionization energy, 11600 kj ⋅ mol−1.
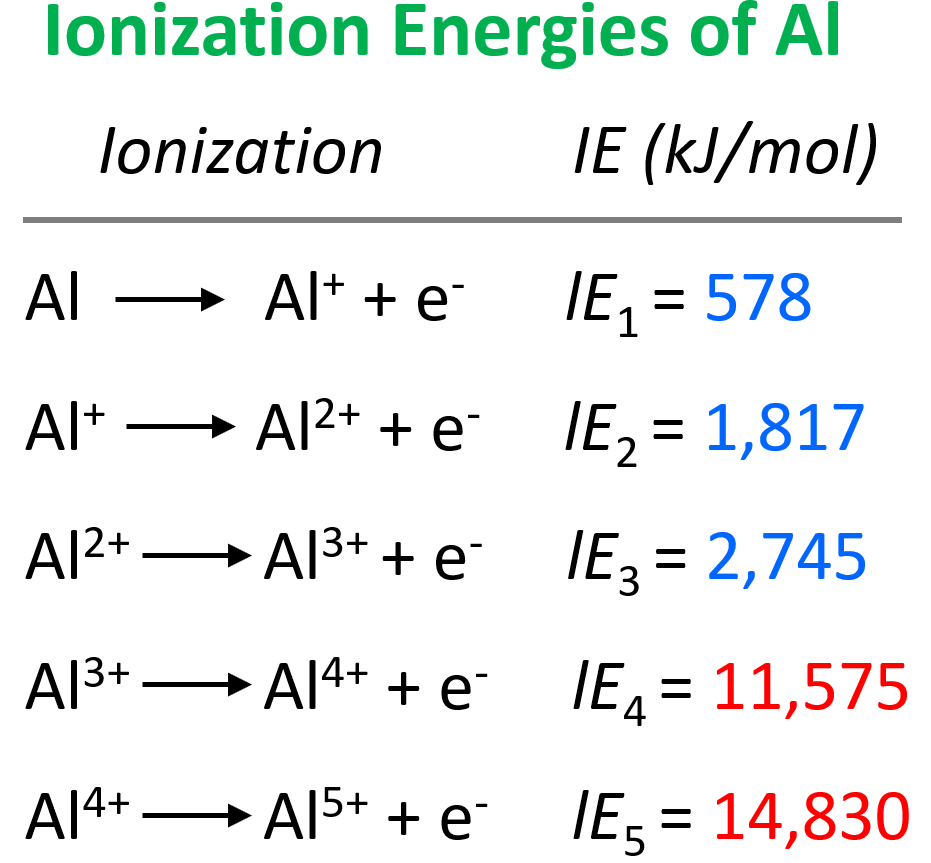
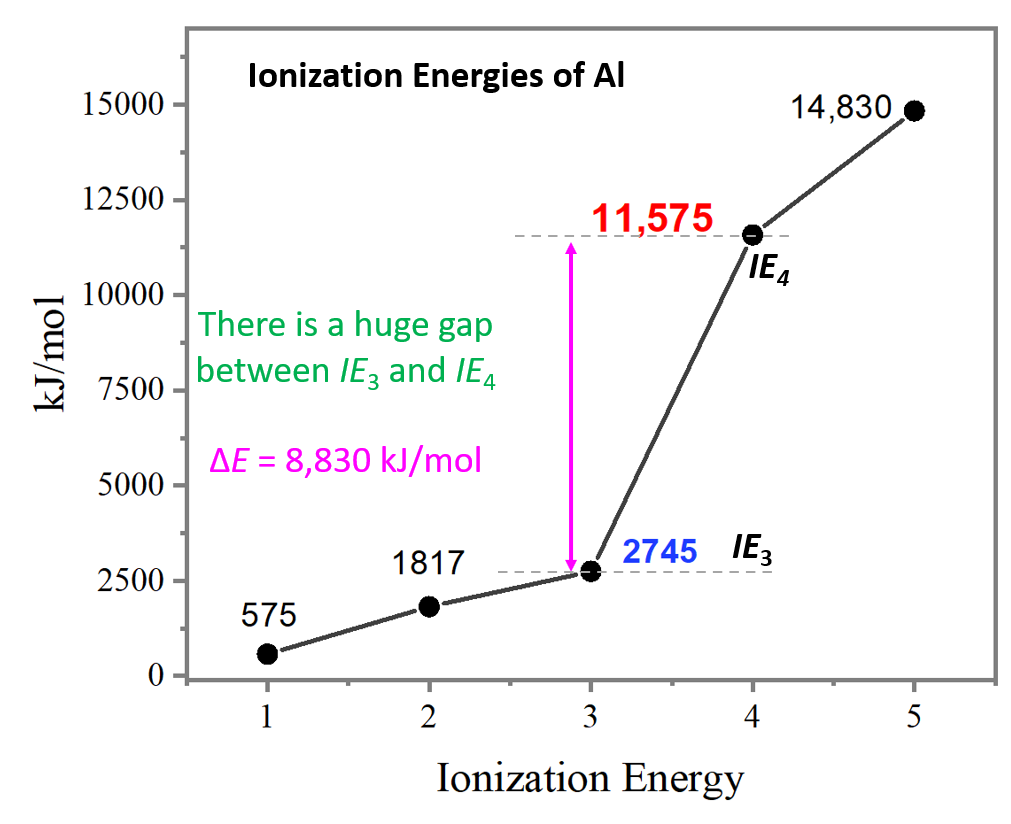
I 4 i_4 i 4 = 11,577 kj/mol By looking for this large jump in energy, we can determine how many valence electrons an. Web we can define a first ionization energy (\(i_1\)), a second ionization energy (\(i_2\)), and in general an \(n^{th}\) ionization energy (\(i_n\)) according to the following reactions: Web to create a successive ionization energy diagram for aluminum, we'll focus on the first few ionization energies: 4th ionization energy, 11600 kj ⋅ mol−1. From the picture, we can see that the fourth ionization energy has a much larger value than the first three energies. For aluminum, this is the 3p electron. That is, it takes more energy to remove the second electron from an atom than the first, and so forth. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. Note that in an a level exam you will not necessarily be shown all the successive ionisation energies of an atom, you may only be shown the first 10 or so, but this should still be.
Web the first ionisation energy is labelled with an arrow. X + energy → x+ + e−. 4th ionization energy, 11600 kj ⋅ mol−1. Shielding effect of inner electrons. I 4 i_4 i 4 = 11,577 kj/mol Both ie 2 for na and ie 3 for al are removing the last core electron from the atom. For strong lines (both in atoms and in ions), it is of the order of unity. Web for example, sc and ga both have three valence electrons, so the rapid increase in ionization energy occurs after the third ionization. Web 1st ionization energy, 577 kj ⋅ mol−1; Paste a picture of the graph below.
Ionization energy Chemistry Steps
Predict the order of increasing energy for the following processes: Web for instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). The first ionization energy is the energy required to remove the outermost (valence) electron. This level was determined by interpolation or extrapolation of known experimental values or by..
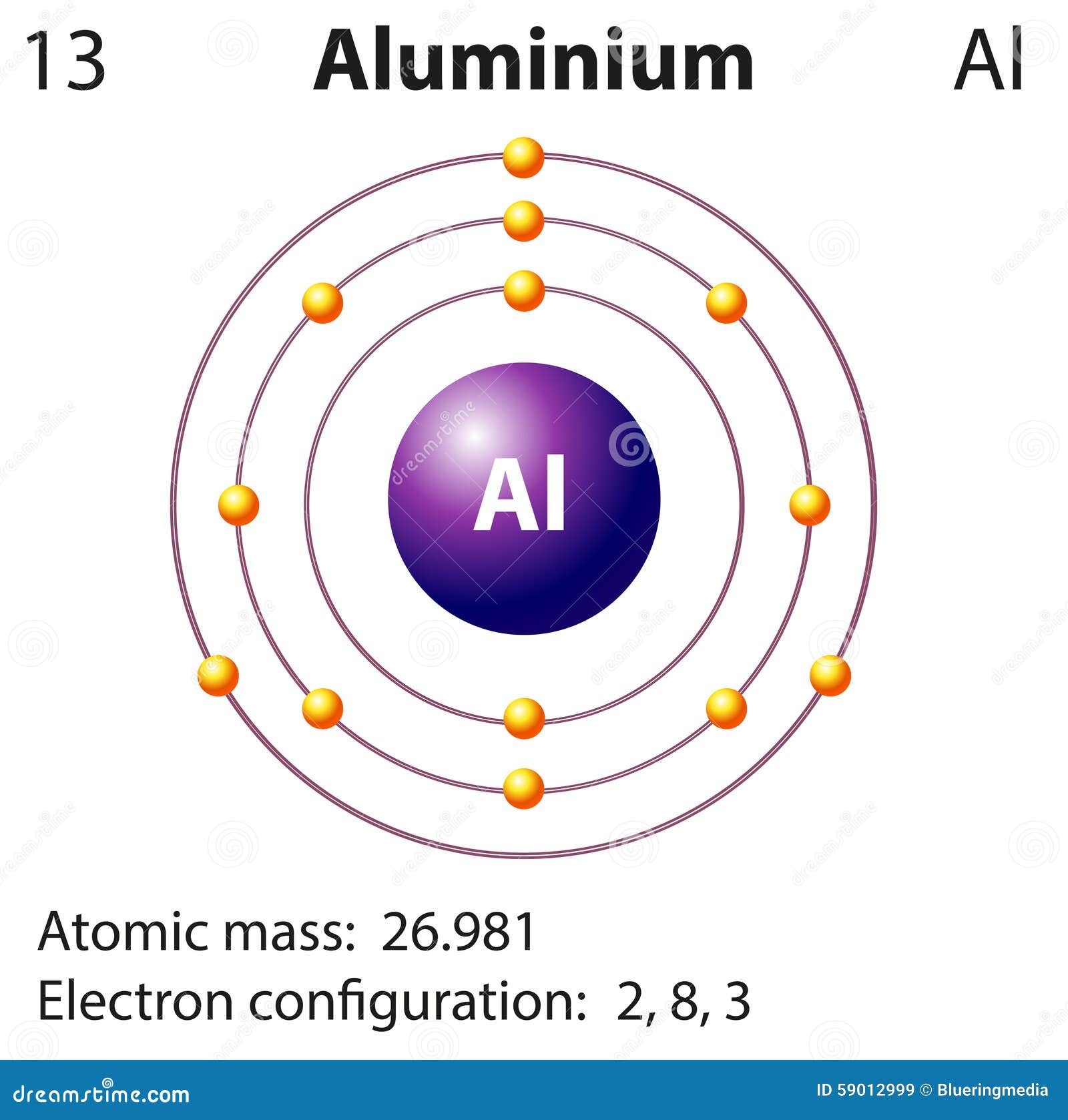
Diagram Representation of the Element Aluminium Stock Vector
I 1 i_1 i 1 = 578 kj/mol. Since these processes will both begin from a cationic state, the electrons will be more difficult to. Web we can define a first ionization energy (i 1), a second ionization energy (i 2), and in general an nth ionization energy (i n) according to the following reactions: Web the size of the.
Atomic structure
Web for example, sc and ga both have three valence electrons, so the rapid increase in ionization energy occurs after the third ionization. Derived the quoted ionization energy by fitting the 2ind 2d terms (n = 3,4,5,6) to a ritz formula. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. For.
Atomic structure
In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Web the size of the first ionisation energy is affected by four factors: Web you can then have as many successive ionisation energies as there are electrons in the original atom. Web to create a successive ionization energy diagram for aluminum, we'll focus on.
Ionisation Energy AS Level Teaching Resources
Electron affinity and electronegativity of aluminum. Edh~n has kindly furnished a new estimate of the intersystem connec· tion, based on more recent data for this isoelectronic sequence. Web for instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). Web 1st ionization energy, 577 kj ⋅ mol−1; X + energy.
Ionization energy Chemistry Steps
Due to this difference in their ionization energy, when they chemically combine they make an ionic bond. Web 1st ionization energy, 577 kj ⋅ mol−1; Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. Distance of outer electrons from the nucleus. Web first ionization energy of aluminium.
Successive Ionisation Energy vigglegiggle
The 7 electrons from the outer shell of the chlorine atom (shown in blue) are the first to be removed. Predict the order of increasing energy for the following processes: The first four ionisation energies of aluminium, for example, are given by. Derived the quoted ionization energy by fitting the 2ind 2d terms (n = 3,4,5,6) to a ritz formula..
12.1 Successive ionisation energies (HL) YouTube
When electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization energy. Distance of outer electrons from the nucleus. Oscillator strength is a dimensionless quantity. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. I 2.
Explaining Successive Ionisation Energies YouTube
Oscillator strength is a dimensionless quantity. First ionization energy of aluminum is 5.9858 ev. Web first ionization energy of aluminium is 5.9858 ev. Due to this difference in their ionization energy, when they chemically combine they make an ionic bond. Web for example, sc and ga both have three valence electrons, so the rapid increase in ionization energy occurs after.
Ionization energy Chemistry Steps
The first ionization energy is the energy required to remove the outermost (valence) electron. Web to draw a successive ionization energy diagram for aluminum, we will use the ionization energy data given on page 60. Web you can then have as many successive ionisation energies as there are electrons in the original atom. Both ie 2 for na and ie.
The 7 Electrons From The Outer Shell Of The Chlorine Atom (Shown In Blue) Are The First To Be Removed.
Web we can define a first ionization energy (\(i_1\)), a second ionization energy (\(i_2\)), and in general an \(n^{th}\) ionization energy (\(i_n\)) according to the following reactions: Both ie 2 for na and ie 3 for al are removing the last core electron from the atom. Identifying an element from successive ionization energies. Distance of outer electrons from the nucleus.
4Th Ionization Energy, 11600 Kj ⋅ Mol−1.
Web m1+e2 is a mix of a and an , both of which occur only between states of the same parity. Web 1st ionization energy, 577 kj ⋅ mol−1; Shielding effect of inner electrons. For aluminum, this is the 3p electron.
Predict The Order Of Increasing Energy For The Following Processes:
3rd ionization energy, 2881 kj ⋅ mol−1. Web the successive ionization energy diagram is shown in the picture below. X + energy → x+ + e−. I 4 i_4 i 4 = 11,577 kj/mol
First Ionisation Energy Increases Across A Period And Decreases Down A Group.
Edh~n has kindly furnished a new estimate of the intersystem connec· tion, based on more recent data for this isoelectronic sequence. Web to draw a successive ionization energy diagram for aluminum, we will use the ionization energy data given on page 60. Size of the nuclear charge. Web first ionization energy of aluminium is 5.9858 ev.