Draw And Label A Water Molecule
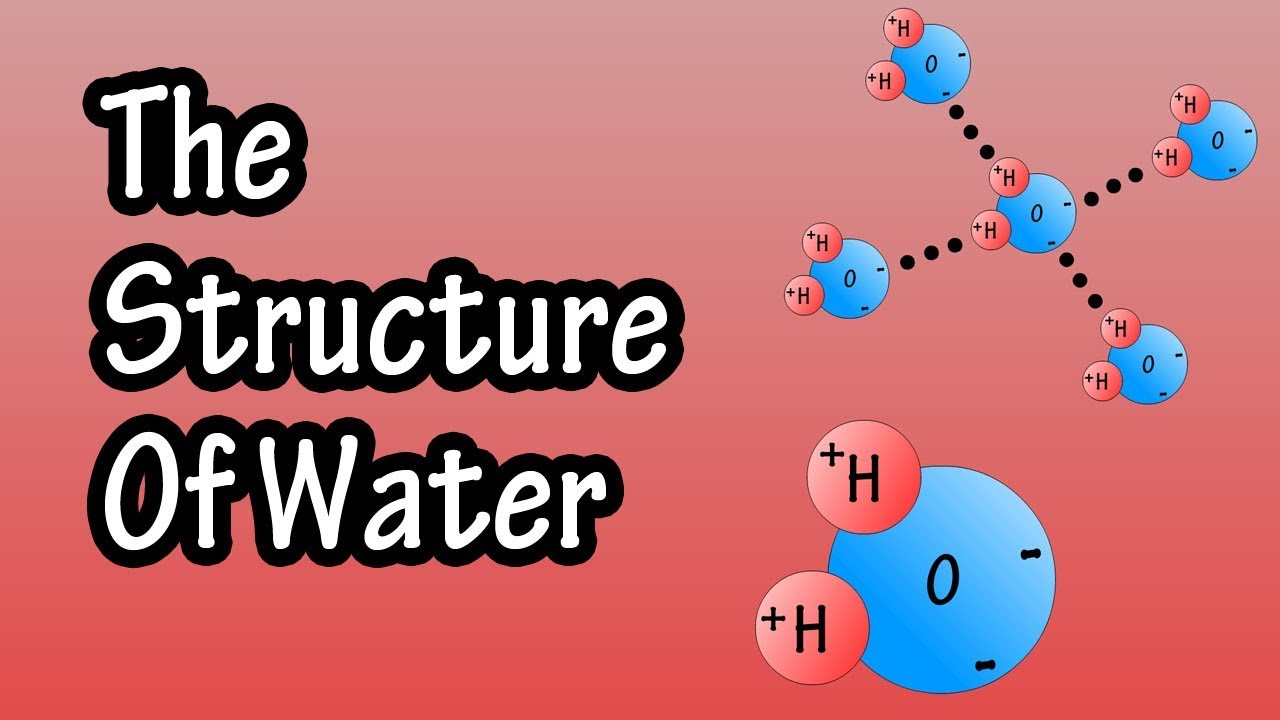
Draw And Label A Water Molecule - Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web with 70% of our earth being ocean water and 65% of our bodies being water, it is hard to not be aware of how important it is in our lives. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. Solid (ice), liquid (water), and gas (steam). Web the water molecule, visualized three different ways: In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the. Remember that a hydrogen atom has one proton and one electron. Your first diagram should be an electron distribution model (showing the atomic nuclei and the electron energy levels). Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding.
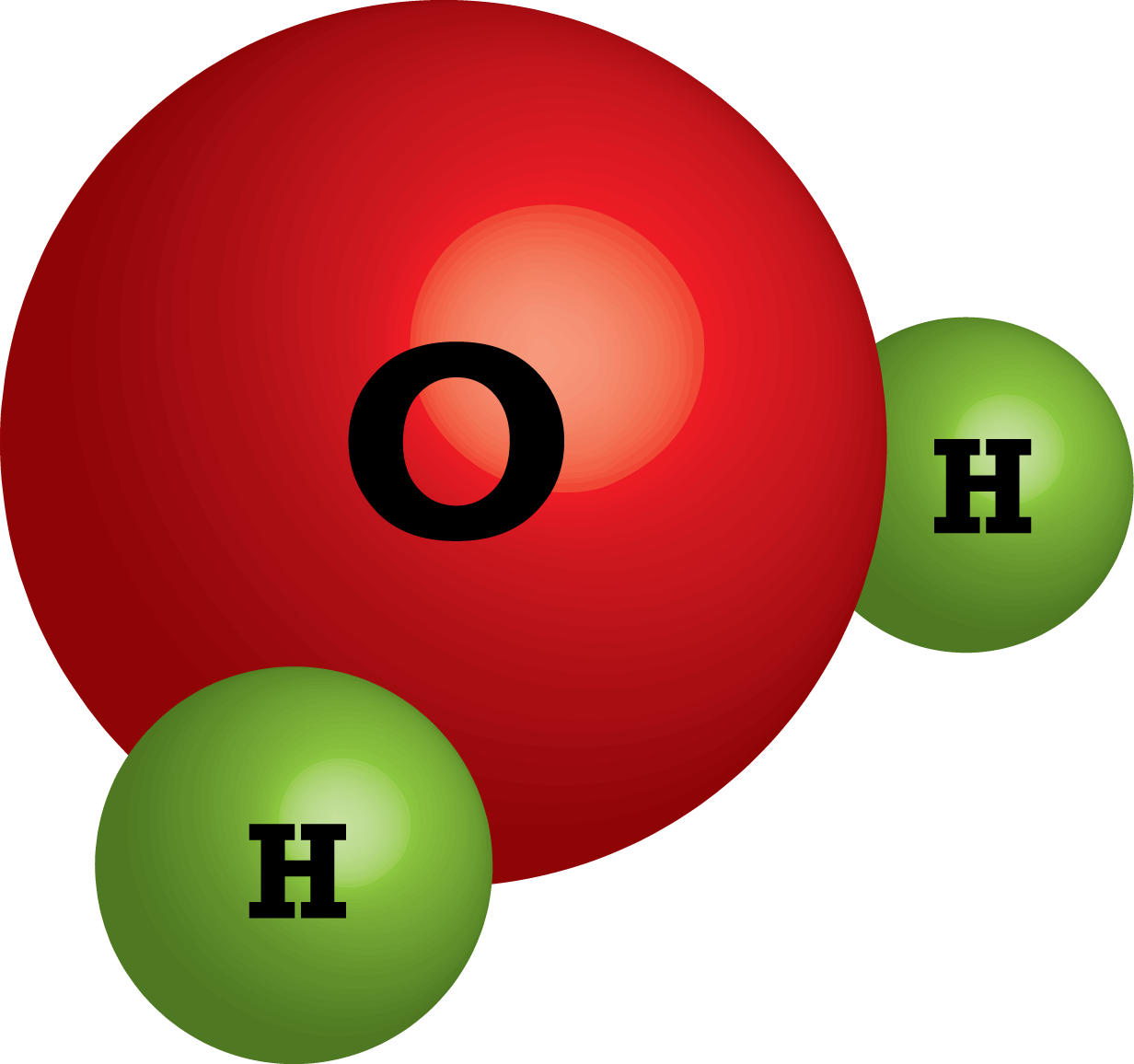
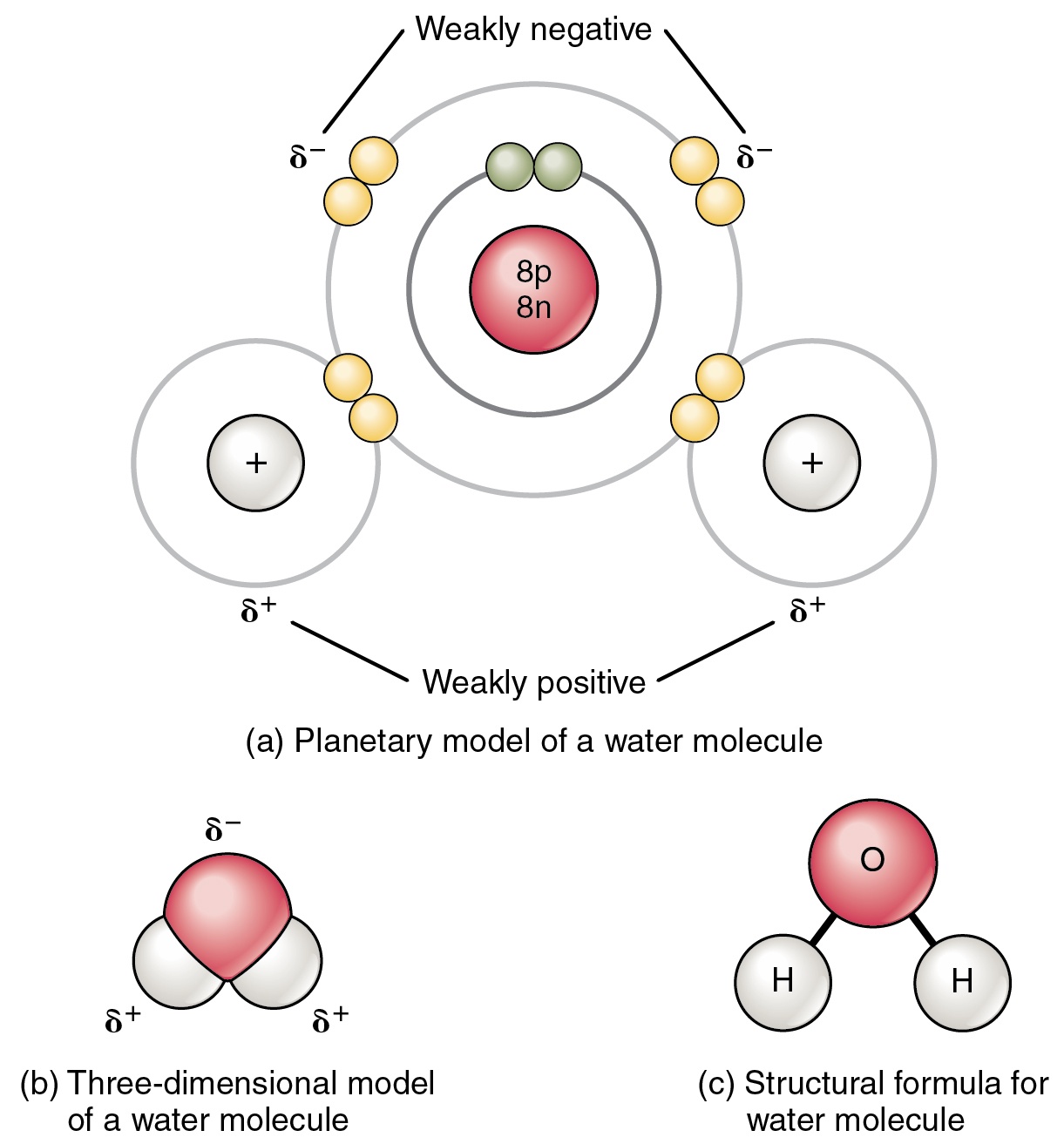
Web to review how to draw a molecule’s structure, let’s start with the simplest molecule in the universe, hydrogen gas (h 2 ). Water molecules are polar, with partial positive charges on the hydrogens, a partial negative charge on the oxygen, and a bent overall structure. Web in a water molecule, the less electronegative hydrogen atoms acquire a partial positive charge and the oxygen atom acquires a partial negative charge. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The diagram below depicts a water molecule. Your first diagram should be an electron distribution model (showing the atomic nuclei and the electron energy levels). Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. The atomic structure of water. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web draw and label a ball and stick presentation of a water molecule.
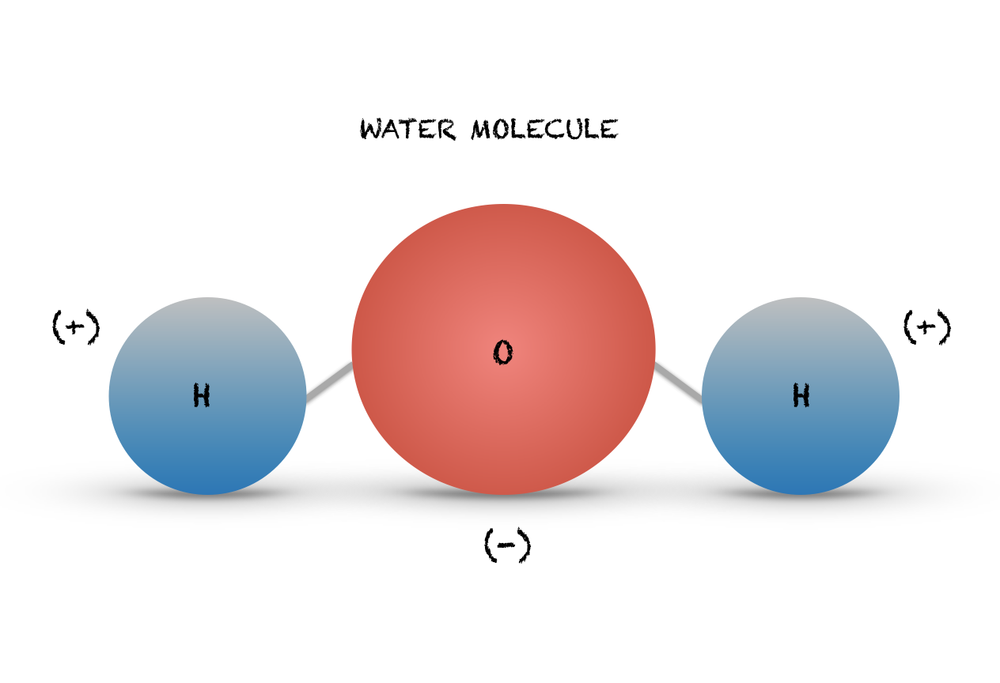
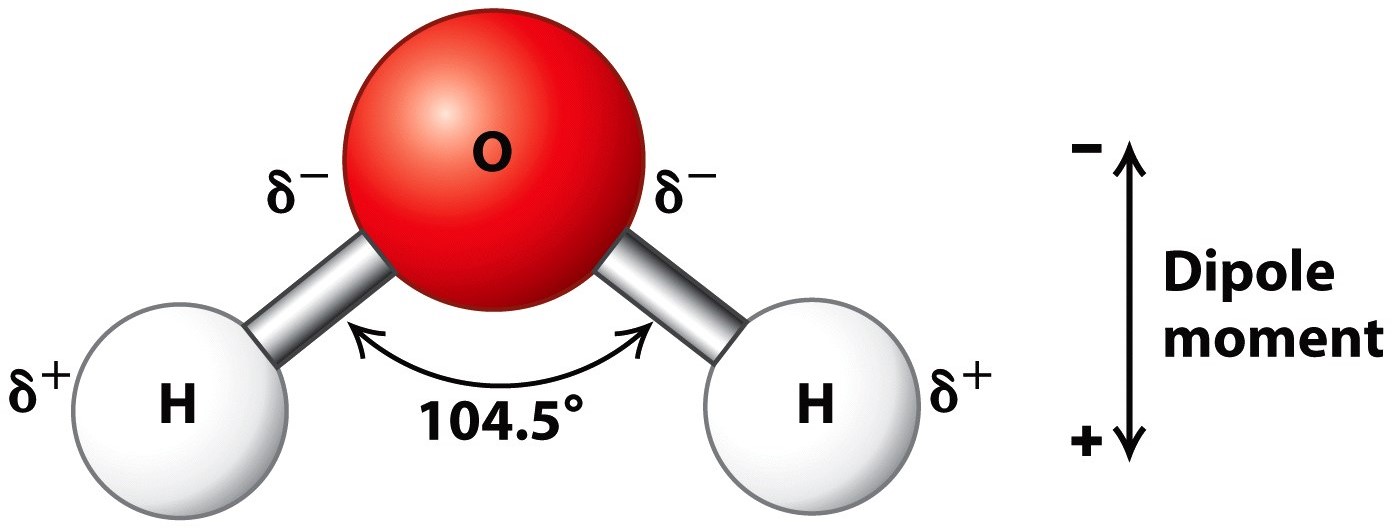
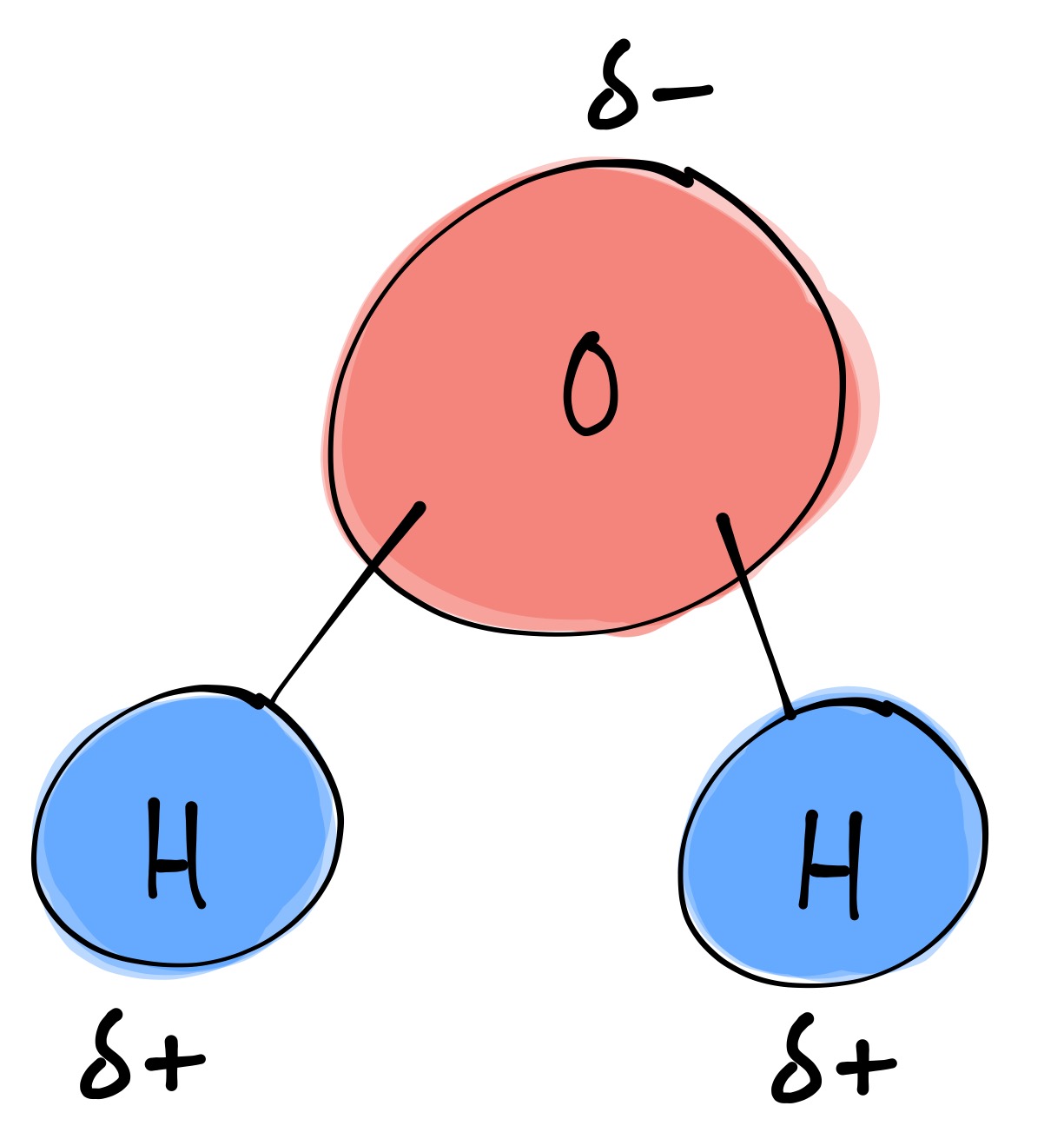
Because the water molecule has an h — o — h bond angle of 105°, the molecule as a whole is polar. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in. Water is an excellent solvent. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). There are two lone pairs of electrons on. Water molecules are polar, with partial positive charges on the hydrogens, a partial negative charge on the oxygen, and a bent overall structure. Web if the water is obtained in the liquid state: Remember that a hydrogen atom has one proton and one electron. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. And the water is obtained in solid form:
Draw a neat well labelled diagram of information of water molecule
Web the water molecule, visualized three different ways: And the water is obtained in solid form: Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. Because of the higher electronegativity of the oxygen atom, the bonds.
32 Draw And Label A Water Molecule Labels 2021
The second should be a. There are 3 different forms of water, or h 2 o: Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. This.
Lewis Structure Of Water
And the water is obtained in solid form: Water molecules are polar, with partial positive charges on the hydrogens, a partial negative charge on the oxygen, and a bent overall structure. Remember that a hydrogen atom has one proton and one electron. Although the water as a whole is electrically neutral, it behaves as an electrical dipole. This is because.
Chemistry model of molecule water H2O scientific elements. Integrated
Because the water molecule has an h — o — h bond angle of 105°, the molecule as a whole is polar. Web molecular structure of water: Learn vocabulary, terms, and more with flashcards, games, and other study tools. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. Because of.
Science online The importance of the water and its structure
The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. Web in a water molecule, the less electronegative hydrogen atoms acquire a partial positive charge and the oxygen atom acquires a partial negative charge. Web molecular structure of water: Start studying label water molecule. Water (h2o) should be drawn as two hydrogen atoms connected.
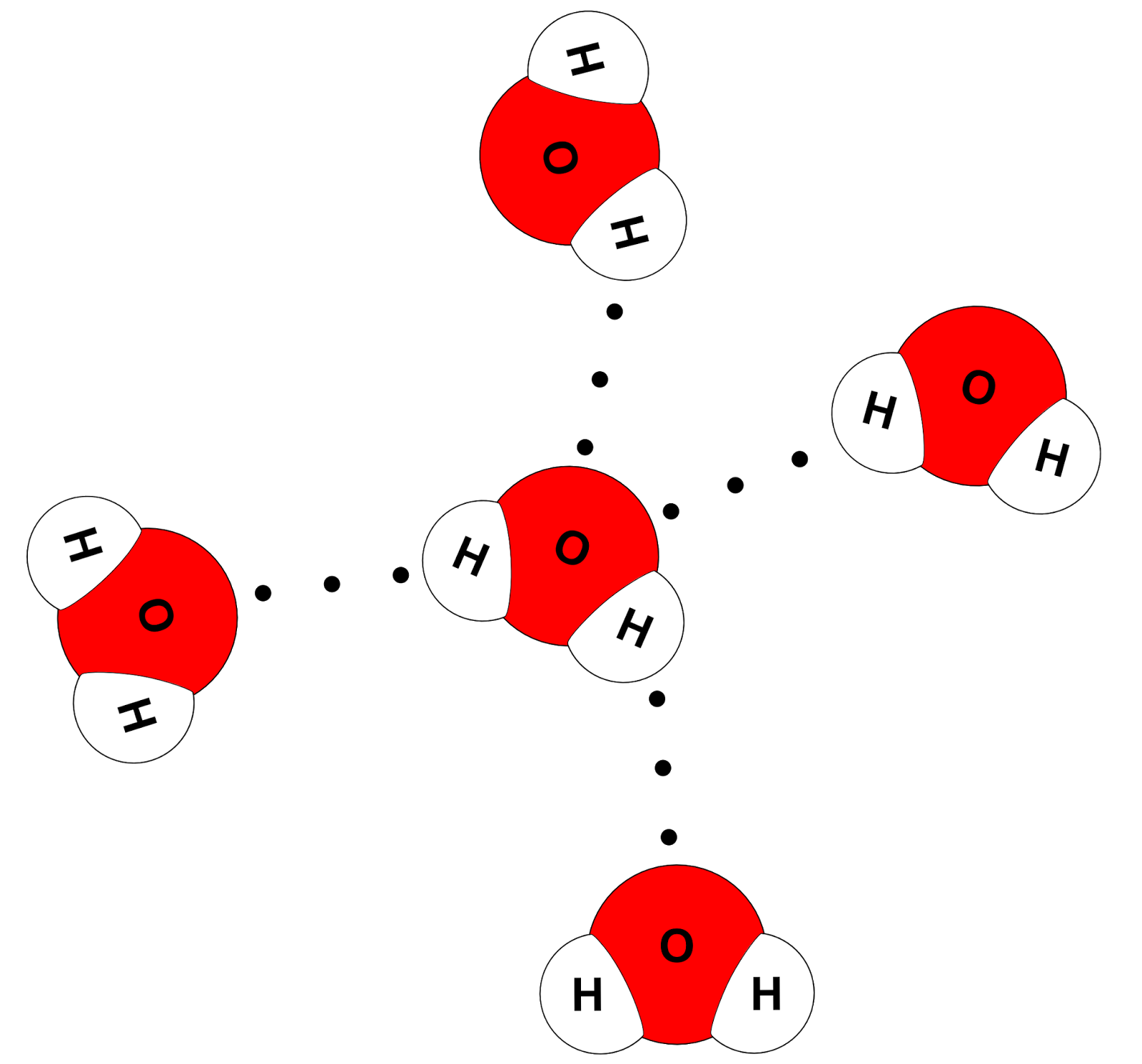
Draw a diagram of water molecules, labeling the hydrogen bond and
Remember that a hydrogen atom has one proton and one electron. Web to review how to draw a molecule’s structure, let’s start with the simplest molecule in the universe, hydrogen gas (h 2 ). The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. In the covalent bond between oxygen and hydrogen, the oxygen.
Structure Of Water Molecule Chemistry Of Water Properties Of Water
Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. In a water molecule, the oxygen atom and hydrogen atoms share electrons in covalent bonds, but the sharing is not equal. Web with 70% of our earth being ocean water and 65% of our bodies being water, it is hard.
Diagram Of Water Molecule Labeled vrogue.co
The one and only electron ring around the nucleus of each hydrogen atom has only one electron. There are 3 different forms of water, or h 2 o: Web if the water is obtained in the liquid state: This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons..
Water — Molecular Structure & Bonding Expii
Web draw a water molecule with charges. In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the. The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. Web the water molecule, visualized three different ways: Describe the structure, such as it is, of liquid water.
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule
Web to review how to draw a molecule’s structure, let’s start with the simplest molecule in the universe, hydrogen gas (h 2 ). Web strong bond within one water molecule when electrons are unequally shared. Web draw and label a ball and stick presentation of a water molecule. The water molecule, as a whole, has 10 protons and 10 electrons,.
First We'll Look At An Mo Diagram For Water, Because It's Pretty Simple And Water Is Very Important.
In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the. Web with 70% of our earth being ocean water and 65% of our bodies being water, it is hard to not be aware of how important it is in our lives. 84k views 11 years ago. The atomic structure of water.
The Surface Molecule Is Attracted To Its Neighbors Below And To Either Side, But There Are No Attractions Pointing In.
Web in a water molecule, the less electronegative hydrogen atoms acquire a partial positive charge and the oxygen atom acquires a partial negative charge. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Water is an excellent solvent. Remember that a hydrogen atom has one proton and one electron.
Web A Molecule Of Water Is Composed Of Two Atoms Of Hydrogen And One Atom Of Oxygen.
Start studying label water molecule. And the water is obtained in solid form: Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons.
The One And Only Electron Ring Around The Nucleus Of Each Hydrogen Atom Has Only One Electron.
Use circles to designate atoms and lines for bonds, then indicate the chemical symbol for each atom. In a water molecule, the oxygen atom and hydrogen atoms share electrons in covalent bonds, but the sharing is not equal. In a water molecule, the oxygen atom and hydrogen atoms share electrons in covalent bonds, but the sharing is not equal. The diagram below depicts a water molecule.