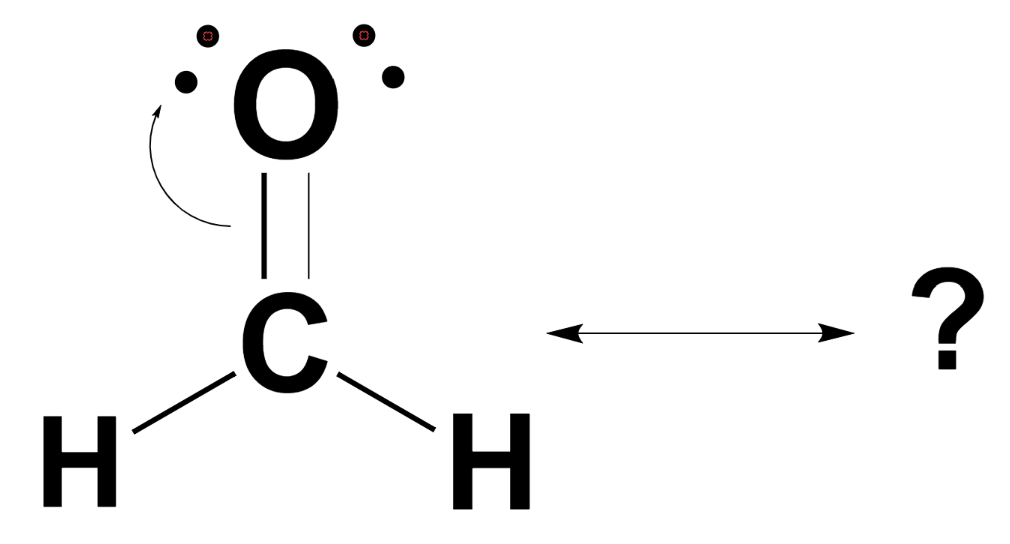
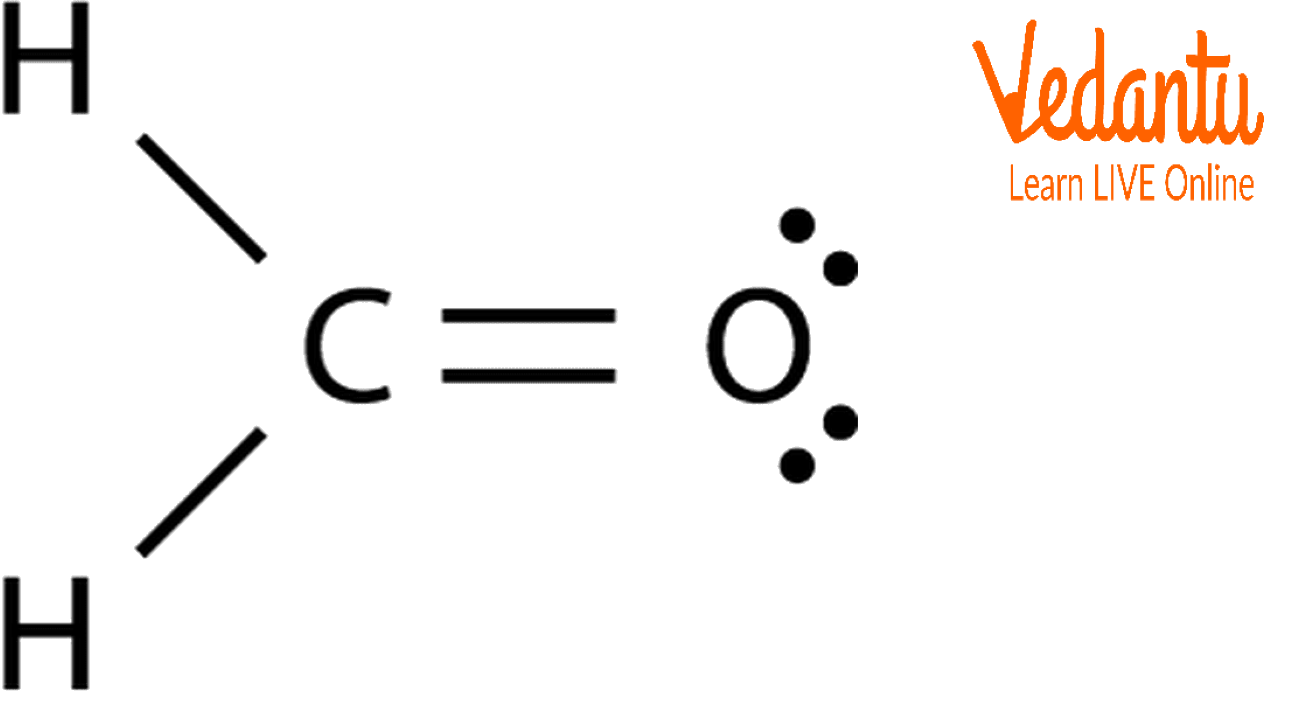Draw Lewis Structure For Ch2O
Draw Lewis Structure For Ch2O - The number of valence electrons for carbon is 4, oxygen is 6, and hydrogen is 1. This video solution was recommended by our tutors as helpful for the problem above. Hybridization of ch 2 o; Determine the total number of electrons in the carbon, hydrogen. Using formal charges to determine how many bonds to make, a different perspective. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This detailed blog on formaldehyde covers all the information related to the compound. A lewis structure is a simplified illustration of the outermost electron configuration of a molecule or ion. 1.) count up the total number of valence electrons available. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms.
It is sometimes referred to as a lewis dot structure or electron dot structure. Web learn the steps to draw the lewis structure of ch2o (formaldehyde) in just 1 minute.📌you can draw any lewis structures by following the simple steps mention. Using formal charges to determine how many bonds to make, a different perspective. Hydrogen, in group 1, has 1 and oxygen, in group 6 or 16, has 6; Web how to draw the lewis structure for ch2o. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. So, the total number of valence electrons in ch2o is: #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure. Web drawing lewis structures for molecules with one central atom: Web let's do the lewis structure for ch2o, methanal or formaldehyde.
Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web how to draw lewis structure of ch 2 o. #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure. How to draw a lewis structure. Web hey guys,in this video, we are going to learn about the lewis structure of ch2o. Drawing lewis structures for bf3, pf3 and brf3; Looking at the periodic table, carbon has 4. Web drawing lewis structures. Based on their placement in the periodic table: Web here’s how you can easily draw the ch 2 o lewis structure step by step:
Lewis Structure Of Ch2o
For the ch2o structure use the periodic table to find the total number of valence electrons for. Determine the total number of valence electrons: How to draw lewis structure of ch 2 o. How to draw a lewis structure. Web drawing lewis structures.
CH2O Lewis Structure, Molecular Geometry, and Hybridization
Here are the steps you need to follow to draw the lewis structure of ch 2 o: In order to draw the lewis structure of ch2o, first of all you have to find the total number of valence electrons present in the ch2o molecule. (1) find the number of valence electrons in the molecule. The number of valence electrons for.
Lewis Structure Of Ch2o
It is a chemical formula for methanol or what is also commonly referred to a. Web hey guys,in this video, we are going to learn about the lewis structure of ch2o. Web drawing lewis structures. Hybridization of ch 2 o; In order to find the total valence electrons in ch2o molecule, first of all you should know the valence electrons.
How to Draw the Lewis Dot Structure for CH2O (Formaldehyde) YouTube
Find the total valence electrons in ch2o molecule. The lewis structure o f the compound is shown in the image attached. Web drawing lewis structures for molecules with one central atom: Web the lewis diagram of a molecule can be constructed using the following stepwise procedure: Hybridization of ch 2 o;
CH2O Lewis Structure Lewis Dot Structure for CH2O Methanal or
Polarity of ch 2 o; #2 mention lone pairs on the atoms. So, the total number of valence electrons in ch2o is: Search for the total already available valence electrons in a single formaldehyde ch2o molecule: The lewis structure o f the compound is shown in the image attached.
CH2O Polar or Nonpolar Learn Important Terms and Concepts
Find more chemistry widgets in wolfram|alpha. But we have two hydrogens, so let's multiply that by 2. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Web this widget gets the lewis structure of chemical compounds. Identify the total number of valence electrons for each atom.
Draw the best Lewis structure for CH2O Based on the Lewis structure for
For the ch2o structure use the periodic table to find the total number of valence electrons for. Web learn the steps to draw the lewis structure of ch2o (formaldehyde) in just 1 minute.📌you can draw any lewis structures by following the simple steps mention. Find the total valence electrons in ch2o molecule. Thus far, we have discussed the various types.
CH2O Lewis Structure How to Draw the Dot Structure for CH2O YouTube
4 (c) + 2 (h) + 6 (o) = 12. The number of valence electrons for carbon is 4, oxygen is 6, and hydrogen is 1. Be the first to ask a question about this topic. Calculate the total number of valence electrons. Using formal charges to determine how many bonds to make, a different perspective.
CH2O Lewis Structure, Molecular Geometry, and Hybridization
Web learn the steps to draw the lewis structure of ch2o (formaldehyde) in just 1 minute.📌you can draw any lewis structures by following the simple steps mention. Web here’s how you can easily draw the ch 2 o lewis structure step by step: (1) find the number of valence electrons in the molecule. Find more chemistry widgets in wolfram|alpha. Web.
lewis structure of ch2o
Send feedback | visit wolfram|alpha. Web how to draw lewis structure of ch 2 o. (2) draw single bonds between bonded atoms. Using formal charges to determine how many bonds to make, a different perspective. Thus far, we have discussed the various types of bonds that form between atoms and/or ions.
Using The Periodic Table To Draw Lewis Dot Structures.
Web how to draw lewis structure for ch 2 o; Carbon (c) is the least electronegative atom in the ch2o lewis structure and therefore.more. Search for the total already available valence electrons in a single formaldehyde ch2o molecule: Be the first to ask a question about this topic.
Web 6 Steps To Draw The Lewis Structure Of Ch2O Step #1:
In order to find the total valence electrons in ch2o molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. Hydrogen, in group 1, has 1 and oxygen, in group 6 or 16, has 6; For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. 1.) count up the total number of valence electrons available.
Breaking The Octet Rule ;
Looking at the periodic table, carbon has 4. 4 (c) + 2 (h) + 6 (o) = 12. So, the total number of valence electrons in ch2o is: Determine the total number of valence electrons:
Hybridization Of Ch 2 O;
#3 if needed, mention formal charges on the atoms. Web hey guys,in this video, we are going to learn about the lewis structure of ch2o. Here are the steps you need to follow to draw the lewis structure of ch 2 o: Web this widget gets the lewis structure of chemical compounds.