Draw Ph Scale
Draw Ph Scale - Web explore the ph scale and learn how to measure the acidity or basicity of various substances with this interactive applet. Water having more hydrogen ion concentration than hydroxyl is acidic. In mathematics, you learned that there are infinite values between 0 and 1, or between 0 and 0.1, or between 0 and 0.01 or between 0 and any small value. Web the ph scale is used to rank solutions in terms of acidity or basicity (alkalinity). You can use ph to quickly determine whether a given aqueous solution is acidic, basic, or neutral. Web the ph scale is a convenient way to represent the acidity or basicity of a solution. As you can see from the ph scale above, pure water has a ph value of 7. Ph more than 7 corresponds to alkaline ph. You can also compare the ph of different solutions and see how adding water affects their ph values. The science ph scale illustration.
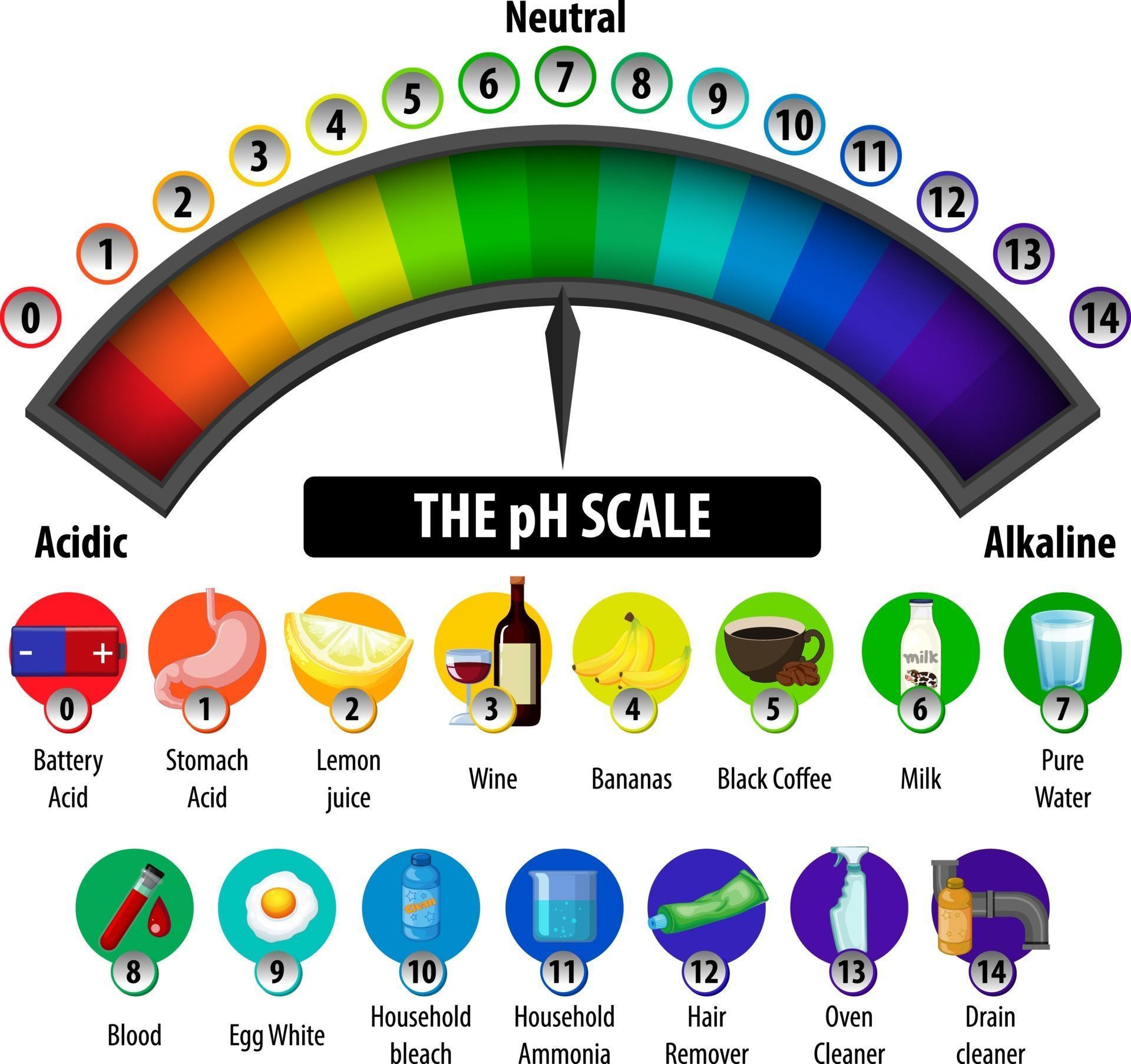
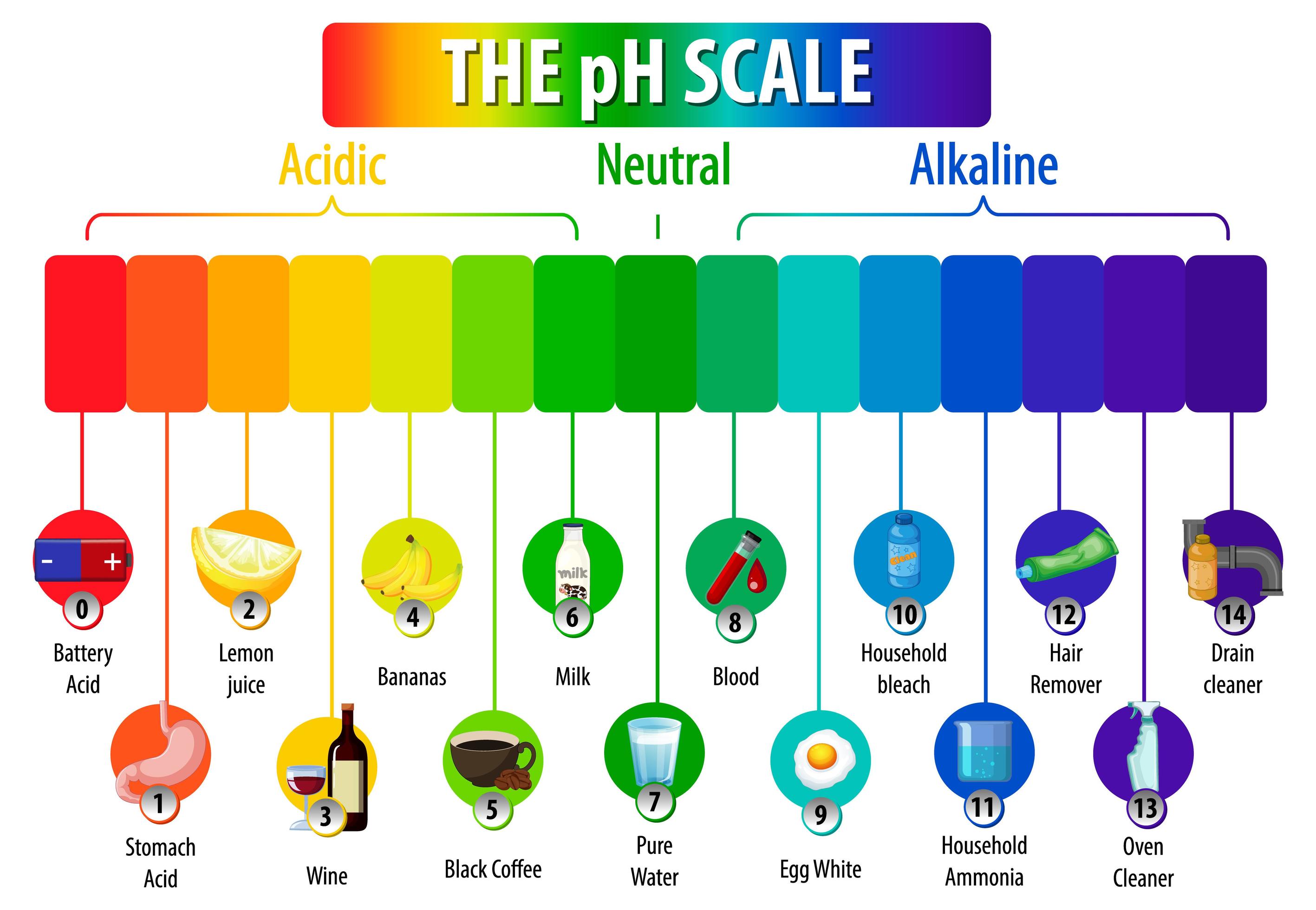
This is known as the ph scale and is the range of values from 0 to 14 that describes the acidity or basicity of a solution. Water having more hydrogen ion concentration than hydroxyl is acidic. Web the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. So chemists defined a new scale that succinctly indicates the concentrations of either of these two ions. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. When pure water is dropped into a solution of universal indicator, the indicator. The science ph scale illustration. Web definitions of ph, poh, and the ph scale. The scale has values ranging from zero (the most acidic) to 14 (the most basic). Ph more than 7 corresponds to alkaline ph.
This is known as the ph scale and is the range of values from 0 to 14 that describes the acidity or basicity of a solution. The ph scale measures how acidic an object is. Web explore math with our beautiful, free online graphing calculator. Web the ph scale is used to rank solutions in terms of acidity or basicity (alkalinity). Web the neat and labeled diagram of ph scale is as shown. Web acidity and alkalinity are measured with a logarithmic scale called ph. In mathematics, you learned that there are infinite values between 0 and 1, or between 0 and 0.1, or between 0 and 0.01 or between 0 and any small value. The scale has values ranging from zero (the most acidic) to 14 (the most basic). Visualize the relative number of hydroxide ions and hydronium ions in solution. Web explore the ph scale and learn how to measure the acidity or basicity of various substances with this interactive applet.
Image result for ph scale drawing Scale drawing, Drawings, Pictures
Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases. The relationship between acid strength and the ph of a solution. Web acidity and alkalinity are measured with a logarithmic scale called ph. Web this is known.
Diagram of the pH scale with examples of acidic, neutral and alkaline
The range of ph is from 0 to 14. For example, a ph of 3 is ten times more acidic than a ph of 4. You can also compare the ph of different solutions and see how adding water affects their ph values. You can measure the ph of common liquids, compare the concentration of hydronium and hydroxide ions, and.
The Science pH Scale 293102 Vector Art at Vecteezy
Web the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. Web if ph > 7, then the solution is basic. You can measure the ph of common liquids, compare the concentration of hydronium and hydroxide ions, and create your own custom solutions. You can also compare the ph.
vector ph scale of acidic,neutral and alkaline value chart for acid and
Web this is known as the \(ph\) scale. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. As we have seen, [h +] and [oh −] values can be markedly different from one aqueous solution to another. Web draw and interpret a ph scale including common acids.
The ph scale diagram 589313 Vector Art at Vecteezy
You can use ph to quickly determine whether a given aqueous solution is acidic, basic, or neutral. Ph 7 corresponds to neutral ph. Web investigate whether changing the volume or diluting with water affects the ph. Web this is known as the \(ph\) scale. Visualize the relative number of hydroxide ions and hydronium ions in solution.
tikz pgf How to draw a pH scale in latex TeX LaTeX Stack Exchange
Calculating the ph of a strong acid or base solution. Web test the ph of things like coffee, spit, and soap to determine whether each is acidic, basic, or neutral. The range of ph is from 0 to 14. The scale has values ranging from zero (the most acidic) to 14 (the most basic). Web the ph scale expands the.
Ph scale diagram with corresponding acidic Vector Image
You can use ph to quickly determine whether a given aqueous solution is acidic, basic, or neutral. Likewise, a ph of 3 is one hundred times more acidic than a ph of 5. Determine the ph of acidic and basic solutions. Here is a table of ph values of common chemicals. Web investigate whether changing the volume or diluting with.
The pH Scale diagram on white background 2988621 Vector Art at Vecteezy
Web acidity and alkalinity are measured with a logarithmic scale called ph. Learn how ph is related to the acidity or basicity of a substance and how it changes with dilution or volume. Ph is a logarithmic function of [h + ]: Water having more hydrogen ion concentration than hydroxyl is acidic. Web explore the ph scale and learn how.
The ph scale diagram 541433 Vector Art at Vecteezy
The range of value s from 0 to 14 that describes the acidity or basicity of a solution. You can measure the ph of common liquids, compare the concentration of hydronium and hydroxide ions, and create your own custom solutions. Visualize the relative number of hydroxide ions and hydronium ions in solution. Here is a table of ph values of.
The pH Scale diagram on white background 1845080 Vector Art at Vecteezy
When pure water is dropped into a solution of universal indicator, the indicator. A strongly acidic solution can have one hundred million million, or one hundred trillion (100,000,000,000,000) times more hydrogen ions than a strongly basic solution! Determine the ph of acidic and basic solutions. Or you can design your own liquid! Web explore the ph scale and learn how.
Web Explore The Ph Scale And Its Applications With This Interactive Simulation.
In mathematics, you learned that there are infinite values between 0 and 1, or between 0 and 0.1, or between 0 and 0.01 or between 0 and any small value. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. You can use \(ph\) to make a quick determination whether a given aqueous solution is acidic, basic, or neutral. Determine the ph of acidic and basic solutions.
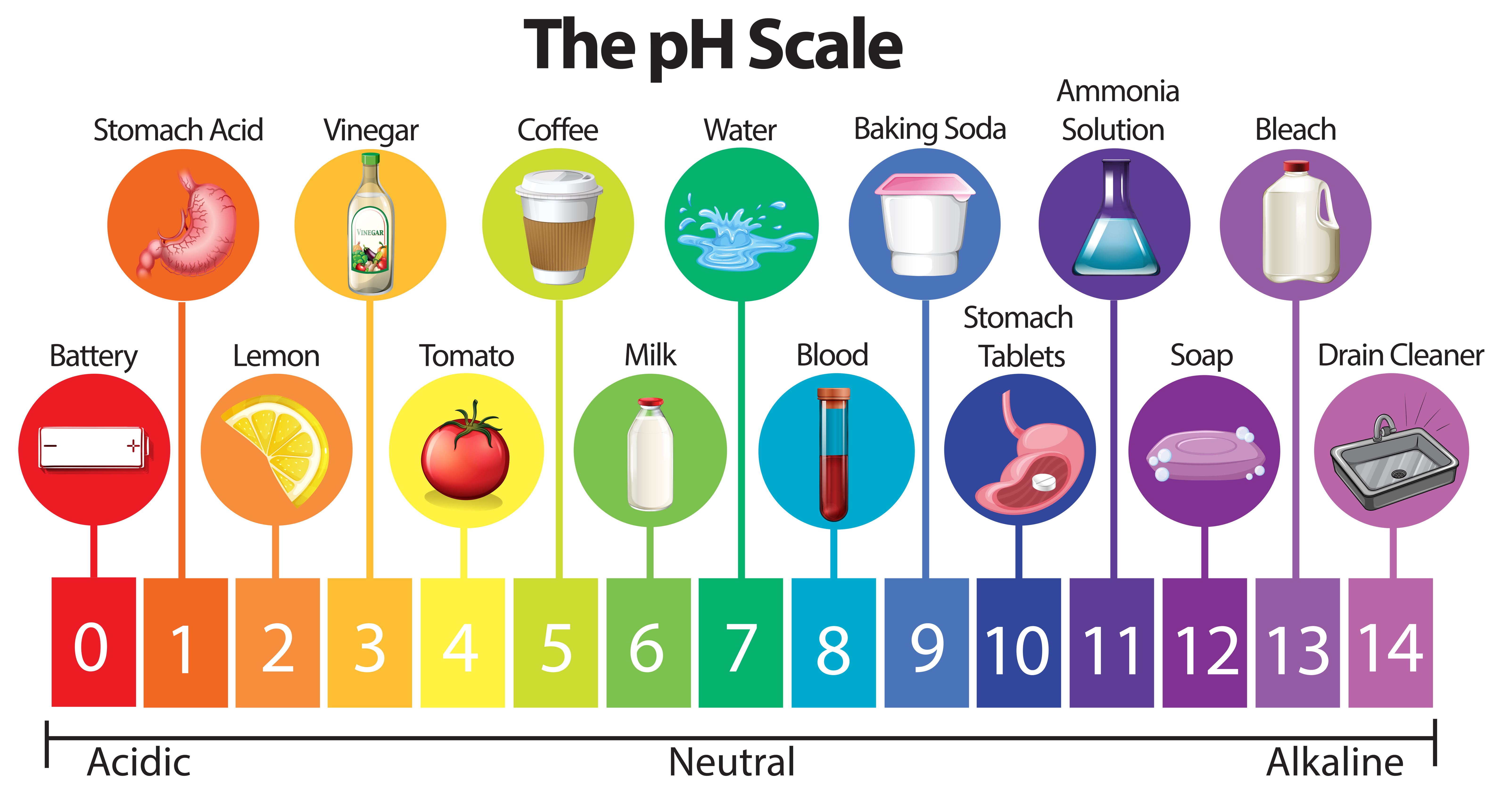
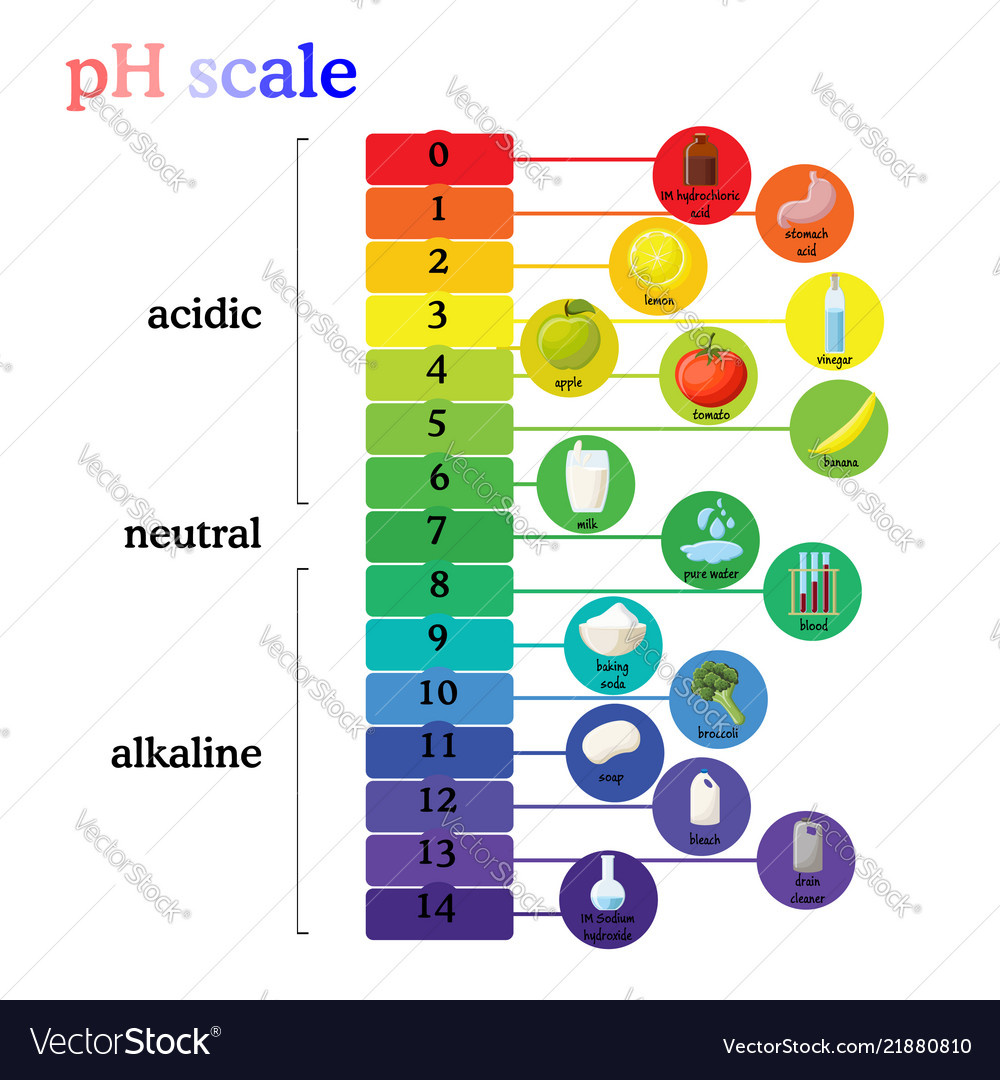
Here Is A Table Of Ph Values Of Common Chemicals.
Web the ph scale is a convenient way to represent the acidity or basicity of a solution. Web the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Web the ph scale is used to rank solutions in terms of acidity or basicity (alkalinity). It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water.
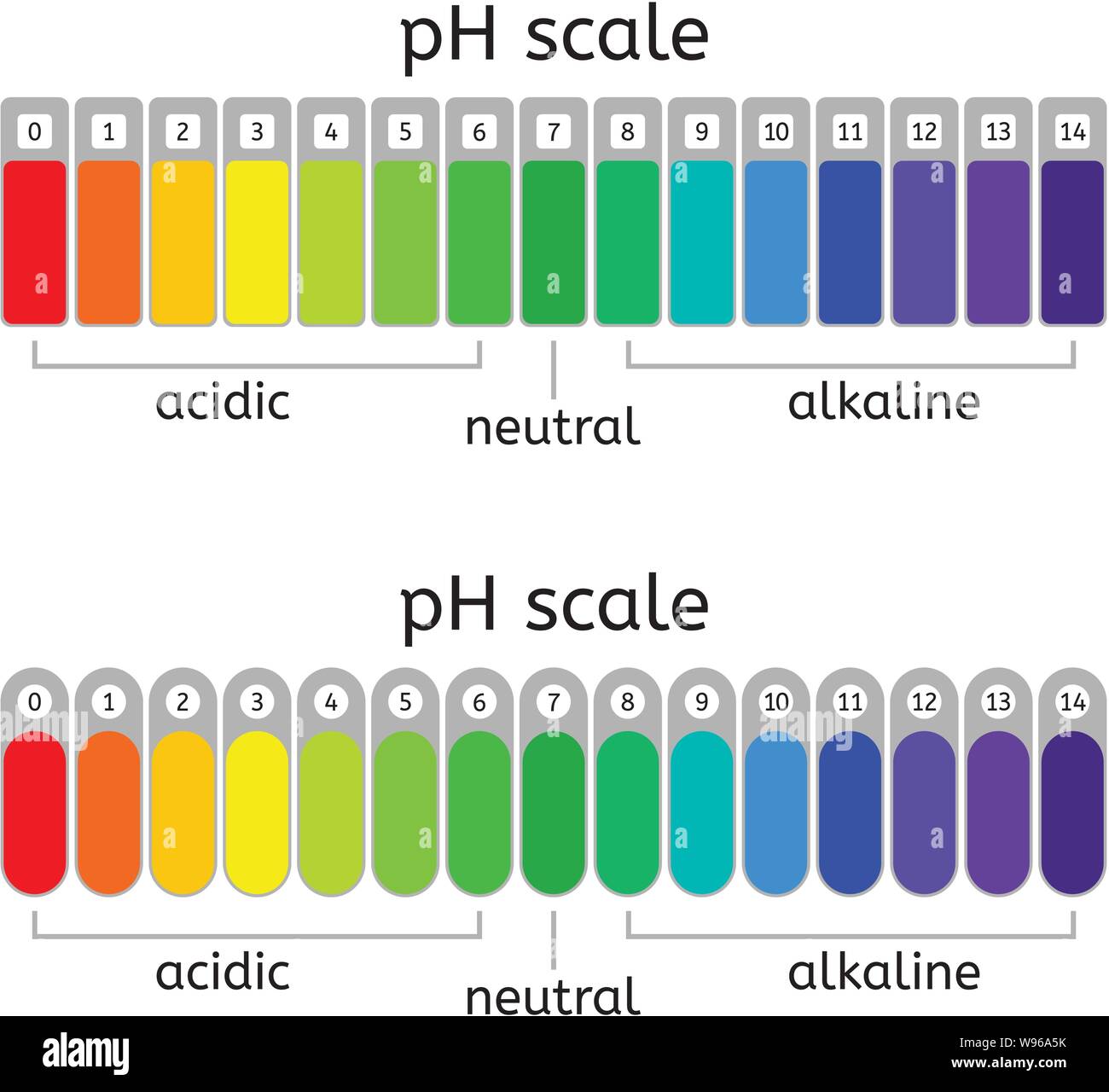
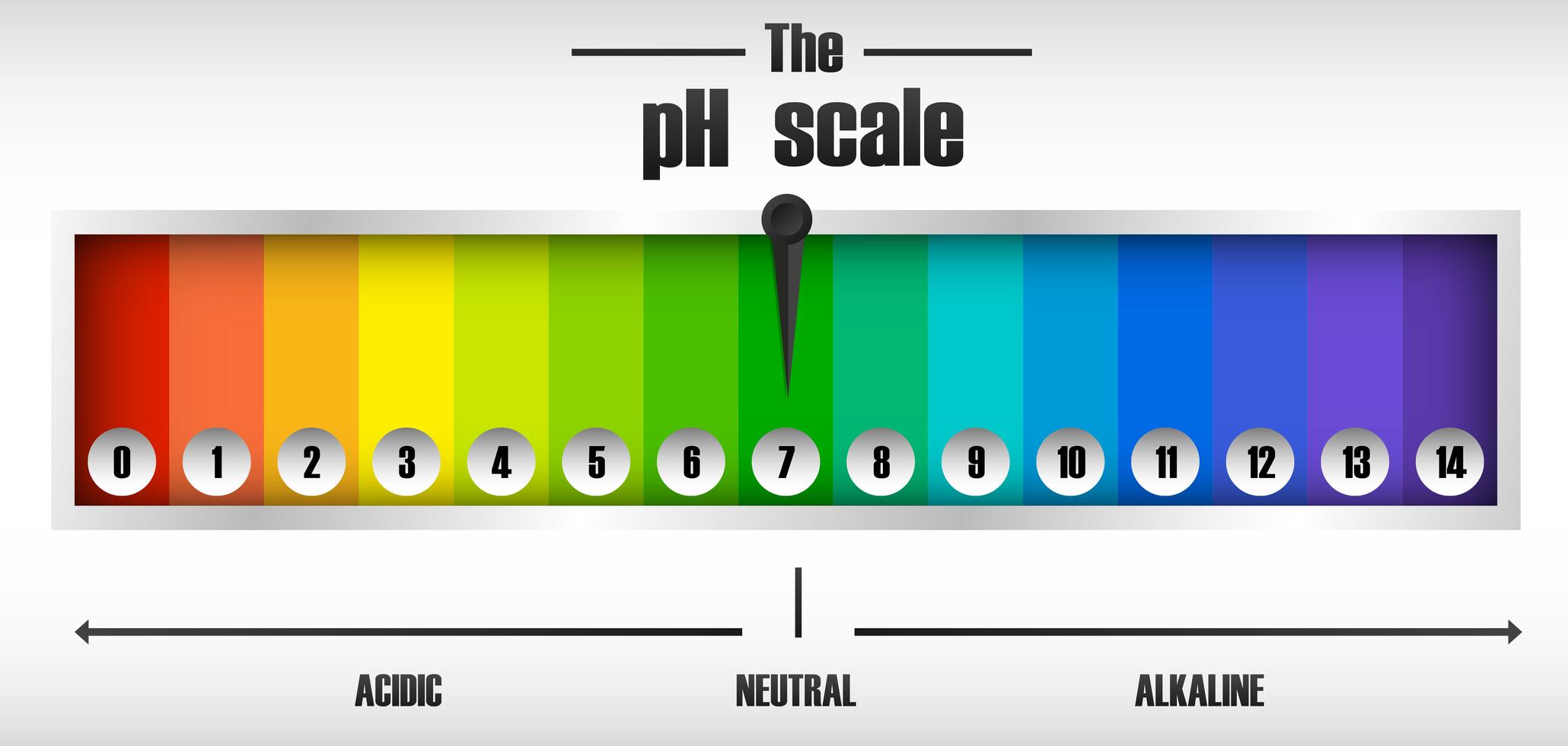
This Is Known As The Ph Scale And Is The Range Of Values From 0 To 14 That Describes The Acidity Or Basicity Of A Solution.
Ph less than 7 corresponds to acidic ph. Calculating the ph of a strong acid or base solution. Visualize the relative number of hydroxide ions and hydronium ions in solution. Ph more than 7 corresponds to alkaline ph.
Or You Can Design Your Own Liquid!
Chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases. The ph scale measures how acidic an object is. Switch between logarithmic and linear scales. For example, a ph of 3 is ten times more acidic than a ph of 4.