Draw The Electron Configuration For A Neutral Atom Of Magnesium
Draw The Electron Configuration For A Neutral Atom Of Magnesium - Web thus, the electron configuration of neutral chlorine atoms is 1s 2 2s 2 2p 6 3s 2 3p 5. The condensed electron configuration is ne 3s2. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Web chemistry electron configuration electron configuration. You can check it here: Group 1a (1), the alkali metals all end is s1. Therefore, the valency and valence electrons of magnesium are 2. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3 s2 configuration, is analogous to its family member beryllium, [he]2 s2. 1s 2 2s 2 2p 6: Energy 1 l x х ?
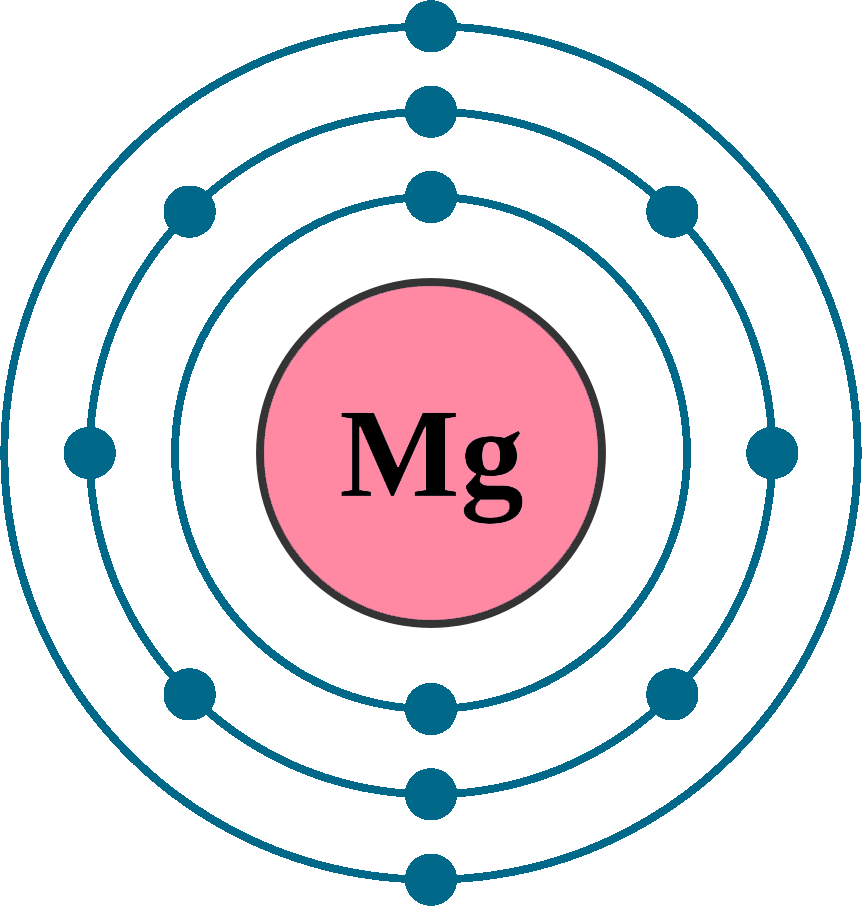
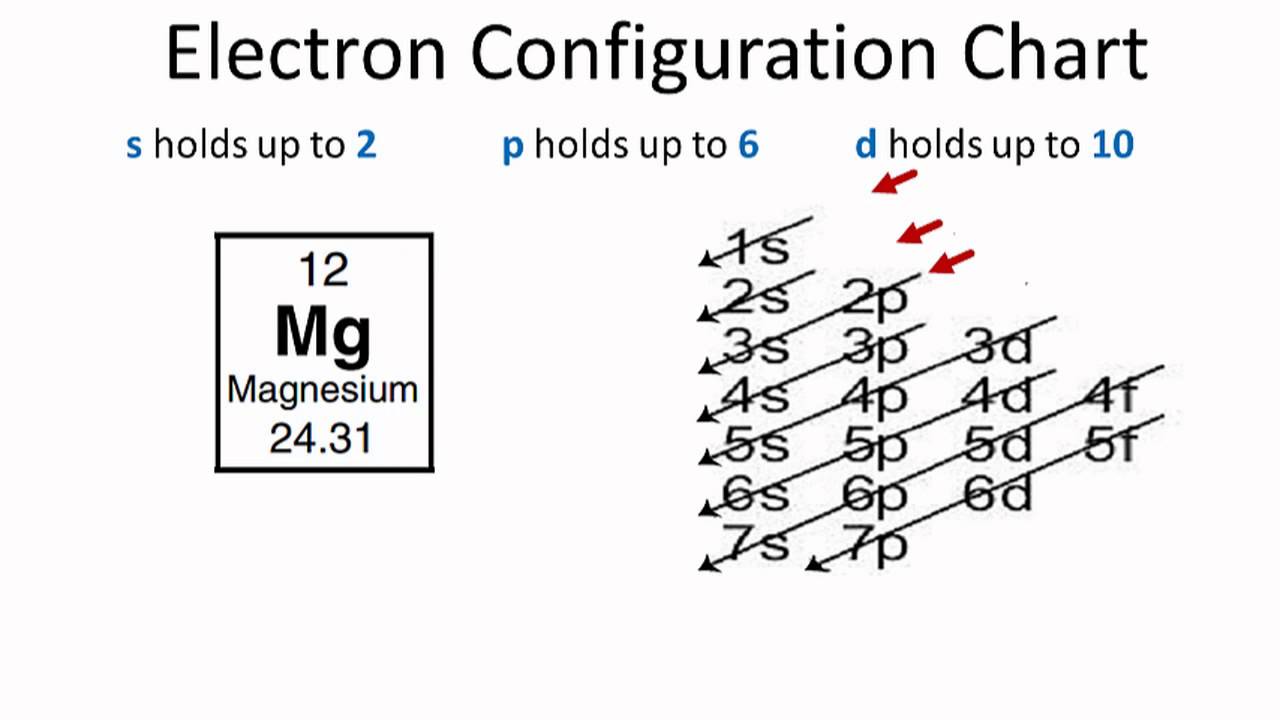
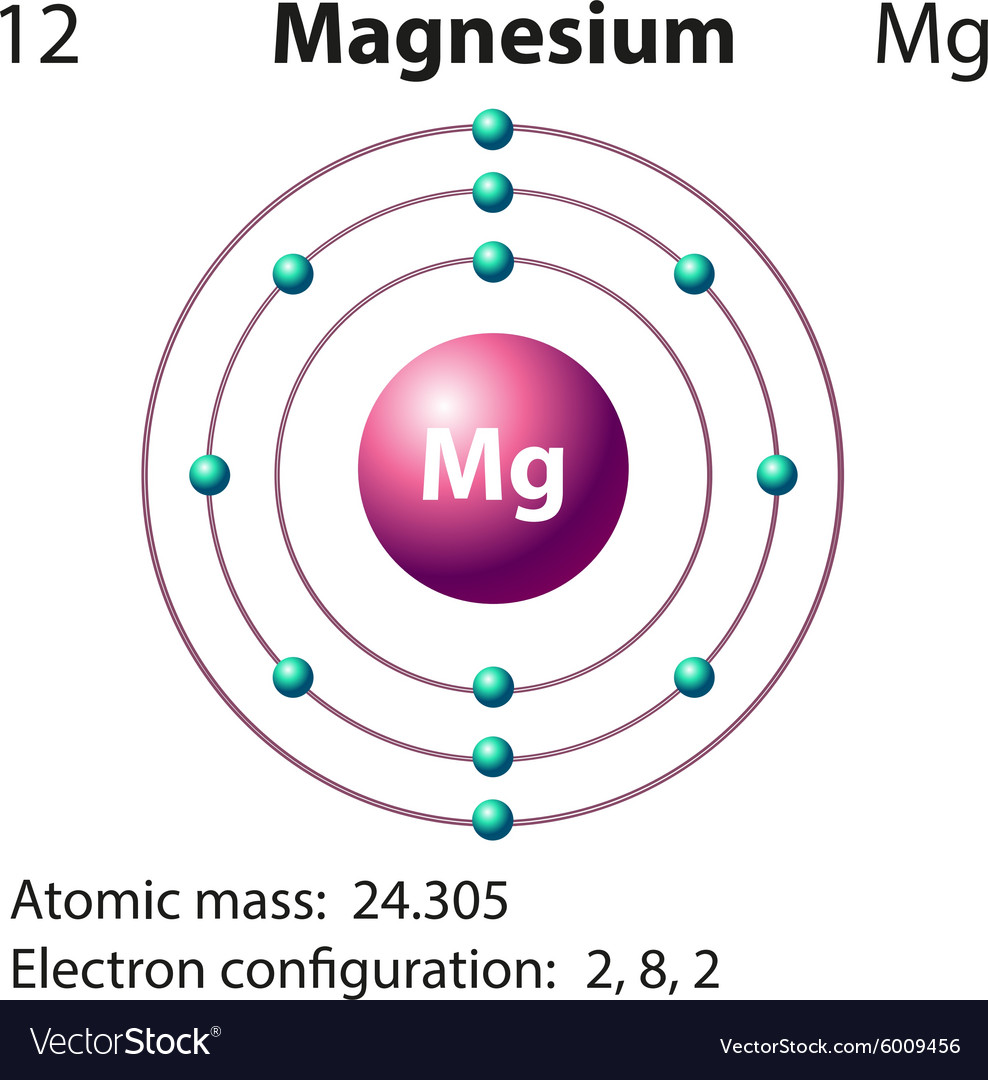
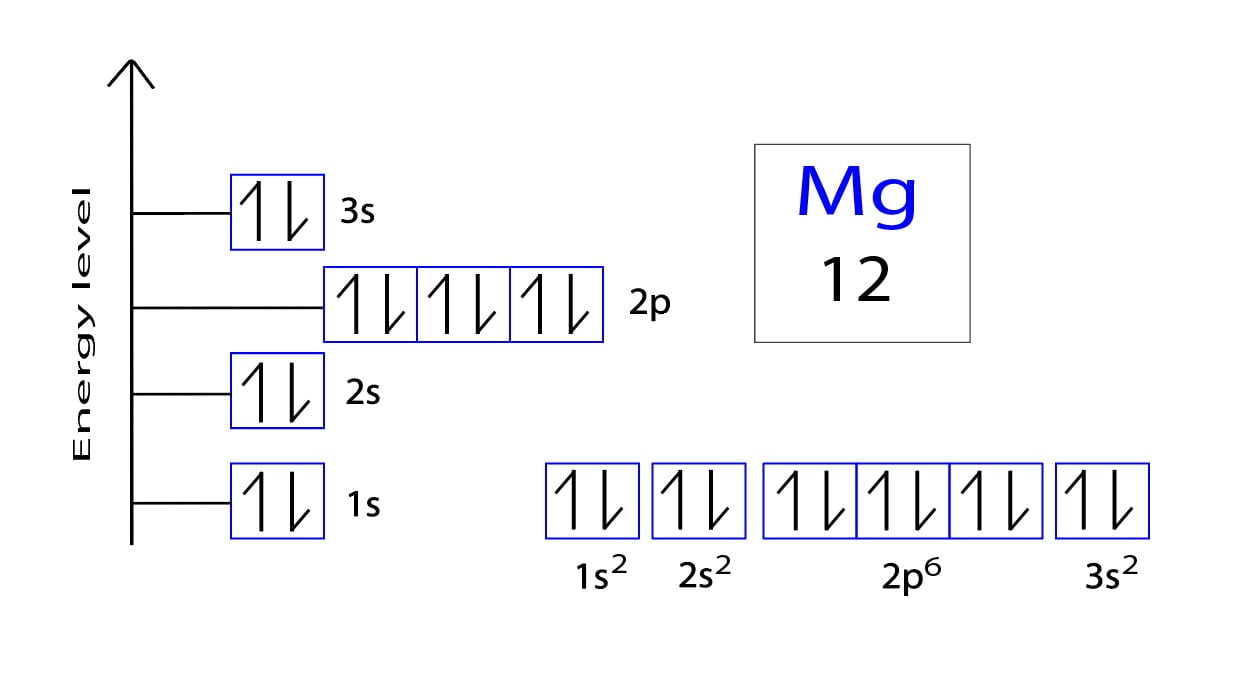
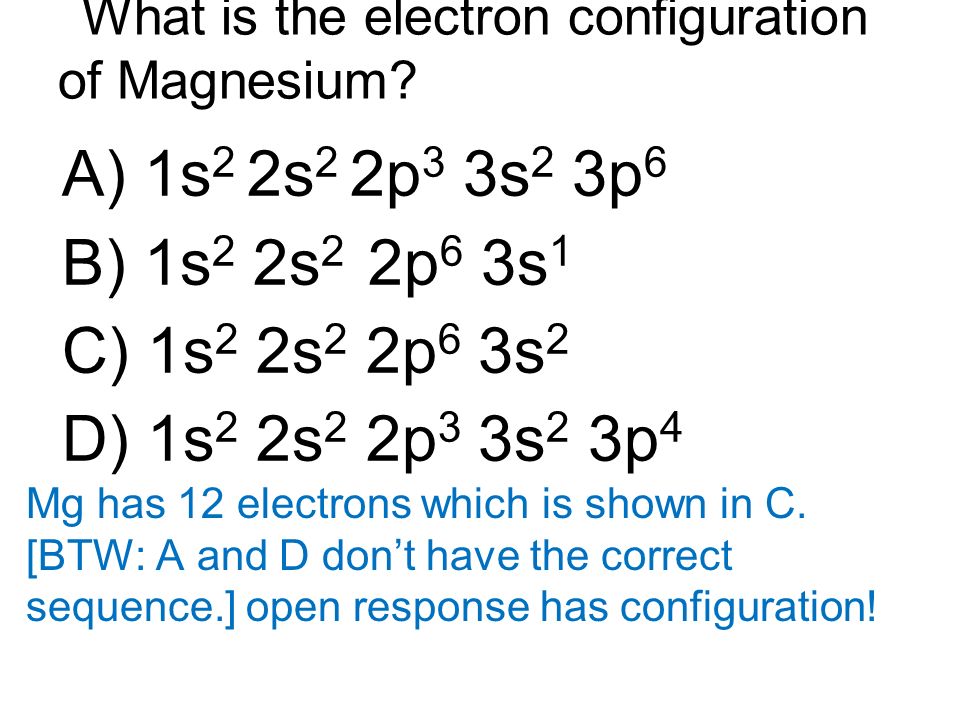
Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Web the valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Web by kirsty patterson 6 september 2021. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. This problem has been solved! Many other electrons configurations of other elements have been provided here for the user. Web ne 3s2 is the electron configuration for mg. Web the ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. 1s 2 2s 2 2p 6: Magnesium is element 12, so it has 12 protons and 12 electrons.
Energy 1 l x х ? You know that magnesium is located in period 3, group 2 of the periodic table, and that it has an atomic number equal to 12. The configuration notation provides an easy way for scientists to write and communicate how. This problem has been solved! 1s 2 2s 2 2p 3: Chemistry bohr model of the atom excited states and ground states. This is the electron configuration of helium; The condensed electron configuration is ne 3s2. Web ne 3s2 is the electron configuration for mg. Electron configuration of oxygen (o) [he] 2s 2 2p 4:
SOLVED Draw the electron configuration for a neutral atom of magnesium
1s 2 2s 2 2p 4: 1s 2 2s 2 2p 6: Understanding the ground state electron configuration of magnesium is crucial in comprehending its. Web the electron configuration of a neutral magnesium atom is: Magnesium is element 12, so it has 12 protons and 12 electrons.
Electron Configuration for Magnesium(Mg, Mg2+ ion)
Web by kirsty patterson 6 september 2021. This problem has been solved! Electron configuration of fluorine (f) [he] 2s 2 2p 5: Web what is the electron configuration of: Web a neutral magnesium atom has an electron configuration of [ne]3s2, implying that it has two electrons in its outermost s orbital.
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for
Draw the electron configuration for a neutral atom of magnesium. 1s 2 2s 2 2p 4: You'll get a detailed solution from a subject matter expert. You know that magnesium is located in period 3, group 2 of the periodic table, and that it has an atomic number equal to 12. Web to write the electron configuration of an atom,.
Magnesium Electron Configuration (Mg) with Orbital Diagram
Web what is the electron configuration of: This video solution was recommended by our tutors as helpful for the problem above. Web your starting point for finding the electron configuration of the magnesiu mcation, mg2+, will be the electron configuration of the neutral magnesium atom, mg. Web both atoms, which are in the alkali metal family, have only one electron.
Atoms Diagrams Electron Configurations of Elements
Web construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web ne 3s2 is the electron configuration for mg. Web what is the electron configuration of: The condensed electron configuration is ne 3s2.
magnesium electron configuration Newton Desk
It denotes a full s orbital. When looking at electron configuration, your fill order of electrons is: Web both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells. Web your starting point for finding the electron configuration of the magnesiu mcation, mg2+, will be the.
Magnesium Electron Configuration YouTube
Web the valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Web the neutral atom chlorine (z=17), for instance has 17 electrons. Chemistry bohr model of the atom excited states and ground states. Understanding the ground state electron configuration of magnesium is crucial in comprehending its. Electron configuration of neon.
Diagram representation of the element magnesium Vector Image
This problem has been solved! Web by kirsty patterson 6 september 2021. Draw the electron configuration for a neutral atom of magnesium. Energy 1 l x х ? Many other electrons configurations of other elements have been provided here for the user.
The magnesium atom with its protons, neutrons, and electrons is
Web both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells. Web by kirsty patterson 6 september 2021. Web the neutral atom chlorine (z=17), for instance has 17 electrons. 1s 2 2s 2 2p 4: This problem has been solved!
Magnesium Atom Science Notes and Projects
Atomic number, atomic weight and charge of magnesium ion. The complete configuration is 1s2 2s2 2p6 3s2. Therefore, the valency and valence electrons of magnesium are 2. You can check it here: Web the neutral atom chlorine (z=17), for instance has 17 electrons.
Web Both Atoms, Which Are In The Alkali Metal Family, Have Only One Electron In A Valence S Subshell Outside A Filled Set Of Inner Shells.
The configuration notation provides an easy way for scientists to write and communicate how. It denotes a full s orbital. The atomic number of magnesium is 12 so we expect the electrons also sum up to 12. This problem has been solved!
Electron Configuration Of Oxygen (O) [He] 2S 2 2P 4:
Understanding the ground state electron configuration of magnesium is crucial in comprehending its. Web the electron configuration of a neutral magnesium atom is: Web construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg. After the electron configuration, the last shell of the magnesium atom has two electrons.
An Electron Configuration Diagram Is A Model That Depicts The Position Of Electrons As They Orbit The Nucleus Of An Atom.
Web ne 3s2 is the electron configuration for mg. 1s 2 2s 2 2p 6: Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. Electron configuration of fluorine (f) [he] 2s 2 2p 5:
Group 1A (1), The Alkali Metals All End Is S1.
For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web therefore the magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Chemistry bohr model of the atom excited states and ground states. The condensed electron configuration is ne 3s2.

:max_bytes(150000):strip_icc()/magnesiumatom-58b6026b5f9b5860464c7467.jpg)