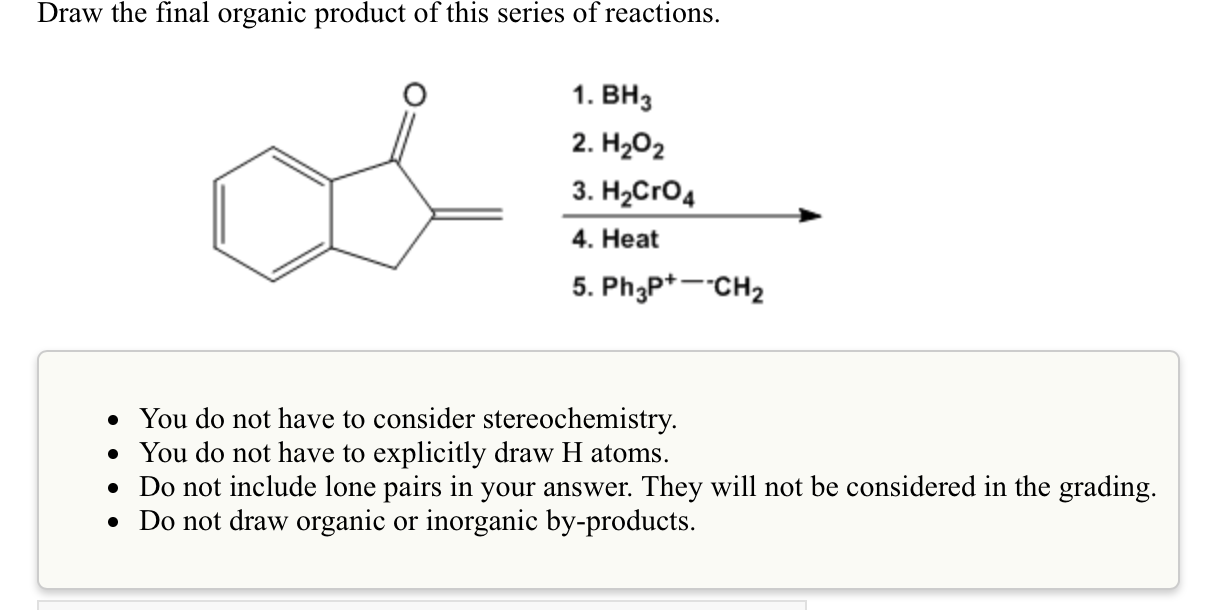
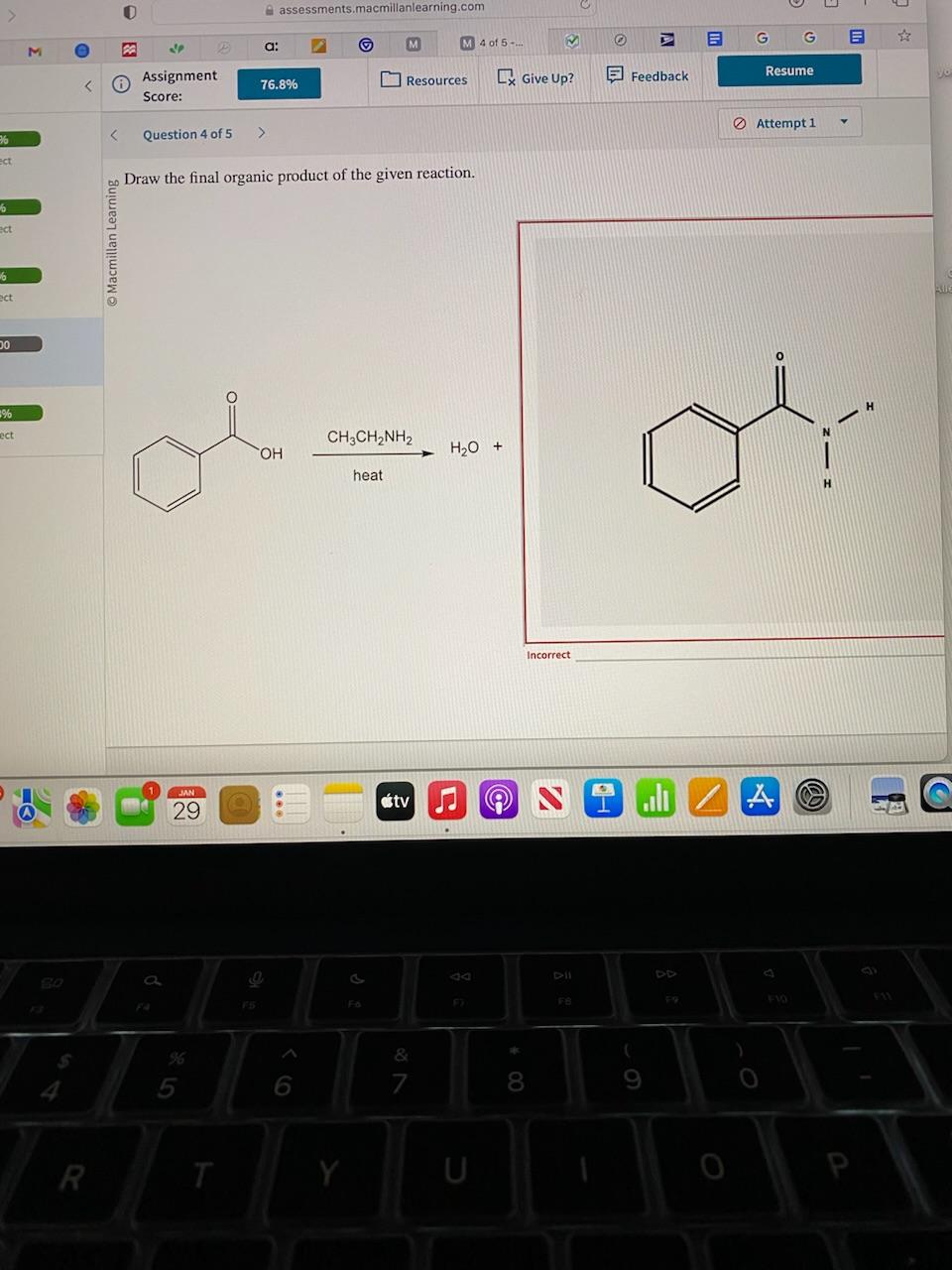
Draw The Final Organic Product Of This Series Of Reactions
Draw The Final Organic Product Of This Series Of Reactions - Aqueous hci you do not have to consider stereochemistry. Web alkene addition reaction with hbr occurs through the pi bond reacting as a nucleophile and abstracting a proton from the acid. This is an extremely common pattern for organic chemistry reactions. 5.0 (11 reviews) click the card to flip 👆. Web here are three examples of nucleophilic substitution reactions. Web the grignard reagent in part (a) reacts with propanal. You do not have to explicitly draw h atoms. • you do not have to explicitly draw h atoms. H2cr04 you do not have to consider stereochemistry. In each case, we are breaking a bond at carbon, and forming a new bond at carbon.
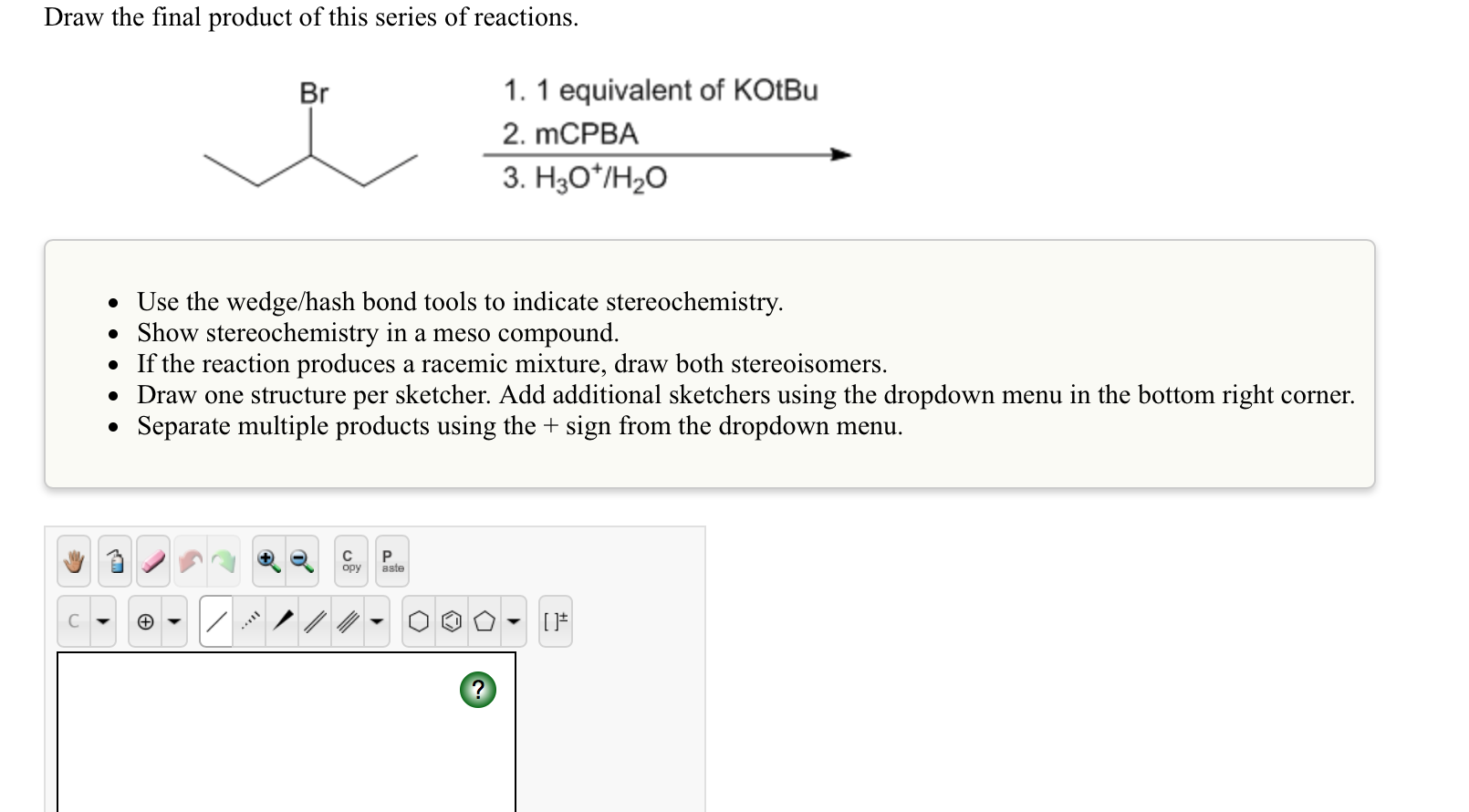
Draw the final organic product of this series of reactions. The final product is an imine. This creates a carbocation intermediate which reacts with the bromide ion to form the final product. You do not have to consider stereochemistry. Do not include lone pairs in your answer. Web for example, if the final product is an alkyl halide, the immediate precursor might be an alkene, to which you could add hx. Web the grignard reagent in part (a) reacts with propanal. When drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect. • • • include the amine from which the enamine was derived. The first reaction is between butyl bromide and hydroxide ion.
Web for example, if the final product is an alkyl halide, the immediate precursor might be an alkene, to which you could add hx. Draw the final organic product of this series of reactions. Predict the organic product of the following reaction. The first reaction is between butyl bromide and hydroxide ion. This creates a carbocation intermediate which reacts with the bromide ion to form the final product. We’ll come back to that in a second. Web alkene addition reaction with hbr occurs through the pi bond reacting as a nucleophile and abstracting a proton from the acid. Reaction rates for this alkene addition reaction increase with larger halogens and more substituted alkenes. You seem very sure about your intermediate b; • you do not have to explicitly draw h atoms.
Solved Draw the final organic product of the reaction.
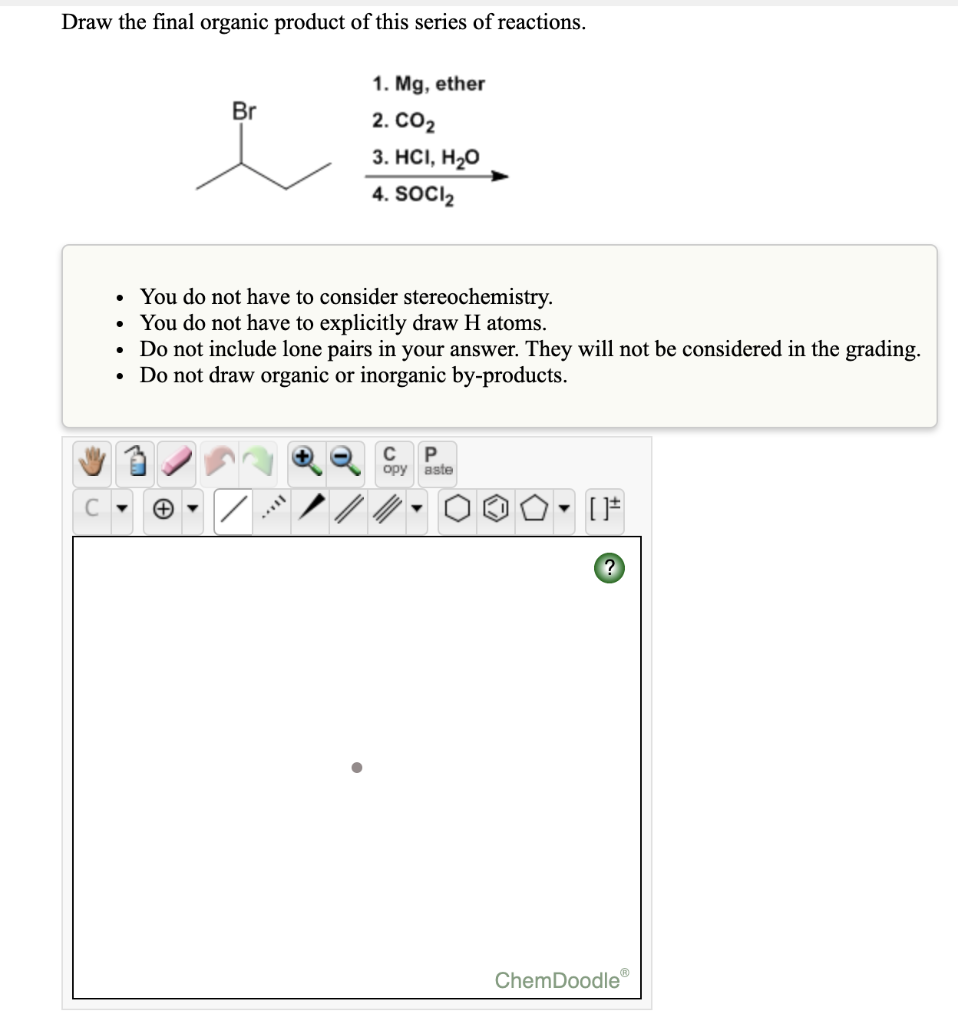
This is an sn2 reaction where the hydroxide ion acts as a nucleophile and attacks the carbon atom attached to the bromine atom. For now, let’s concentrate on the reagents for your final step. If no reaction occurs, draw the organic starting material. You seem very sure about your intermediate b; Soci2 • you do not have to consider stereochemistry.
Solved Draw the final organic product of this series of
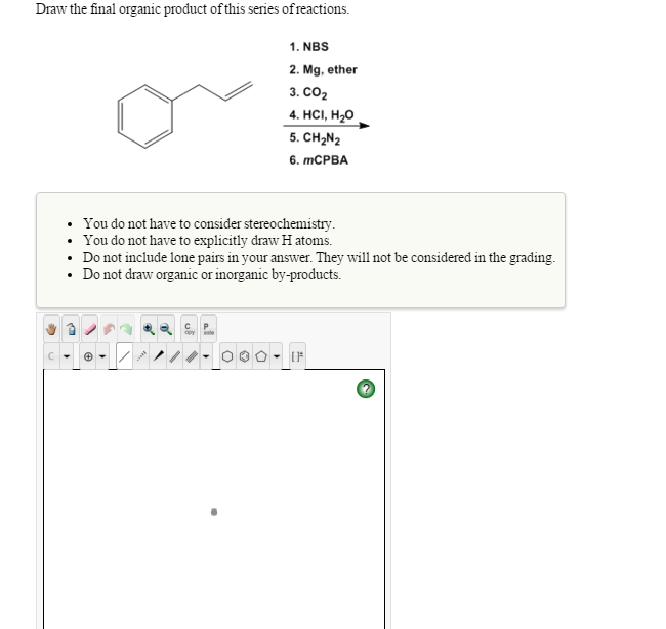
Web the grignard reagent in part (a) reacts with propanal. Web draw the final organic product of this series of reactions. They will not be considered in the grading. You do not have to consider stereochemistry. In this lesson, you will learn about several general categories of organic reactions.
Solved Draw the final organic product of this series of
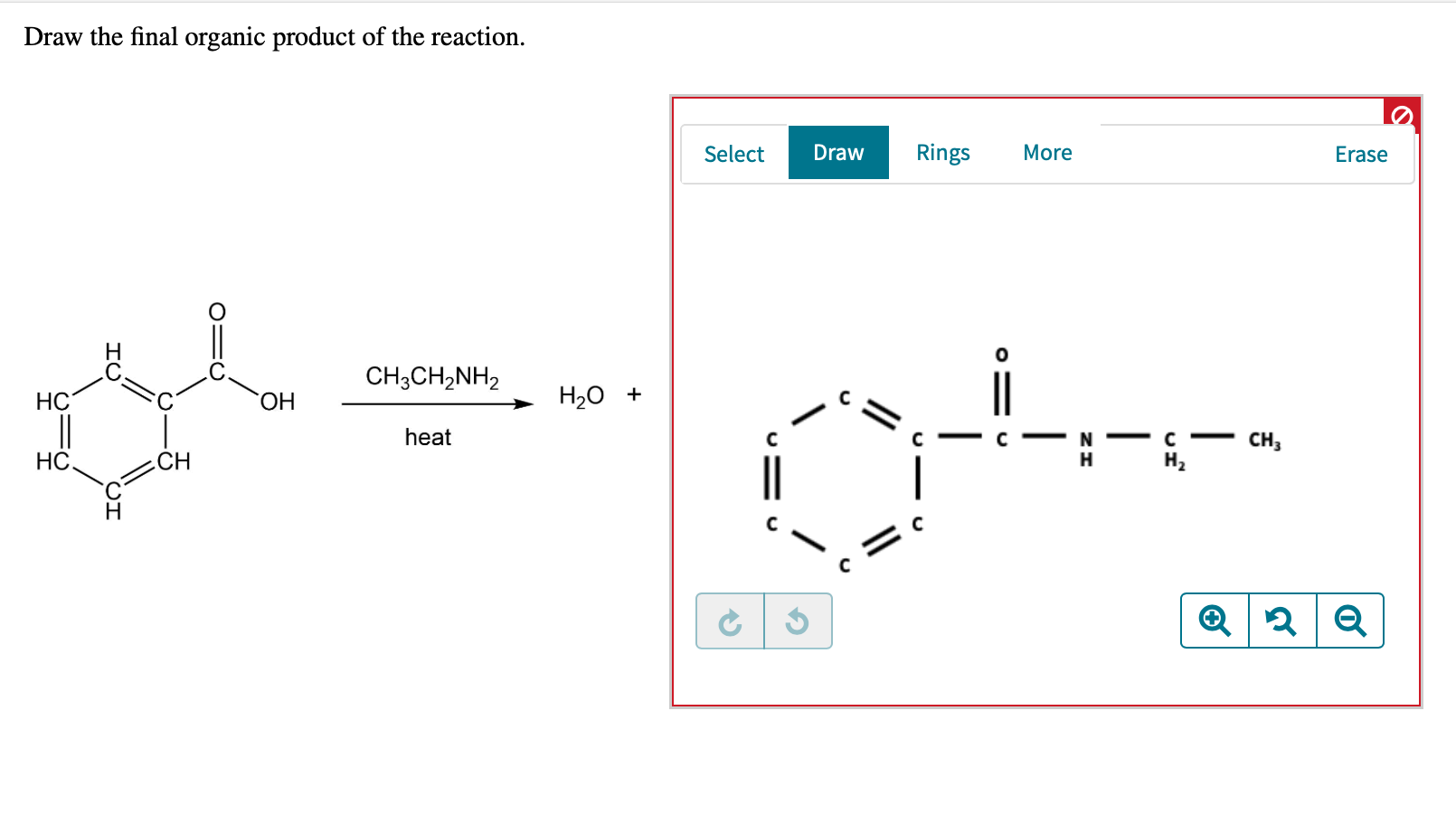
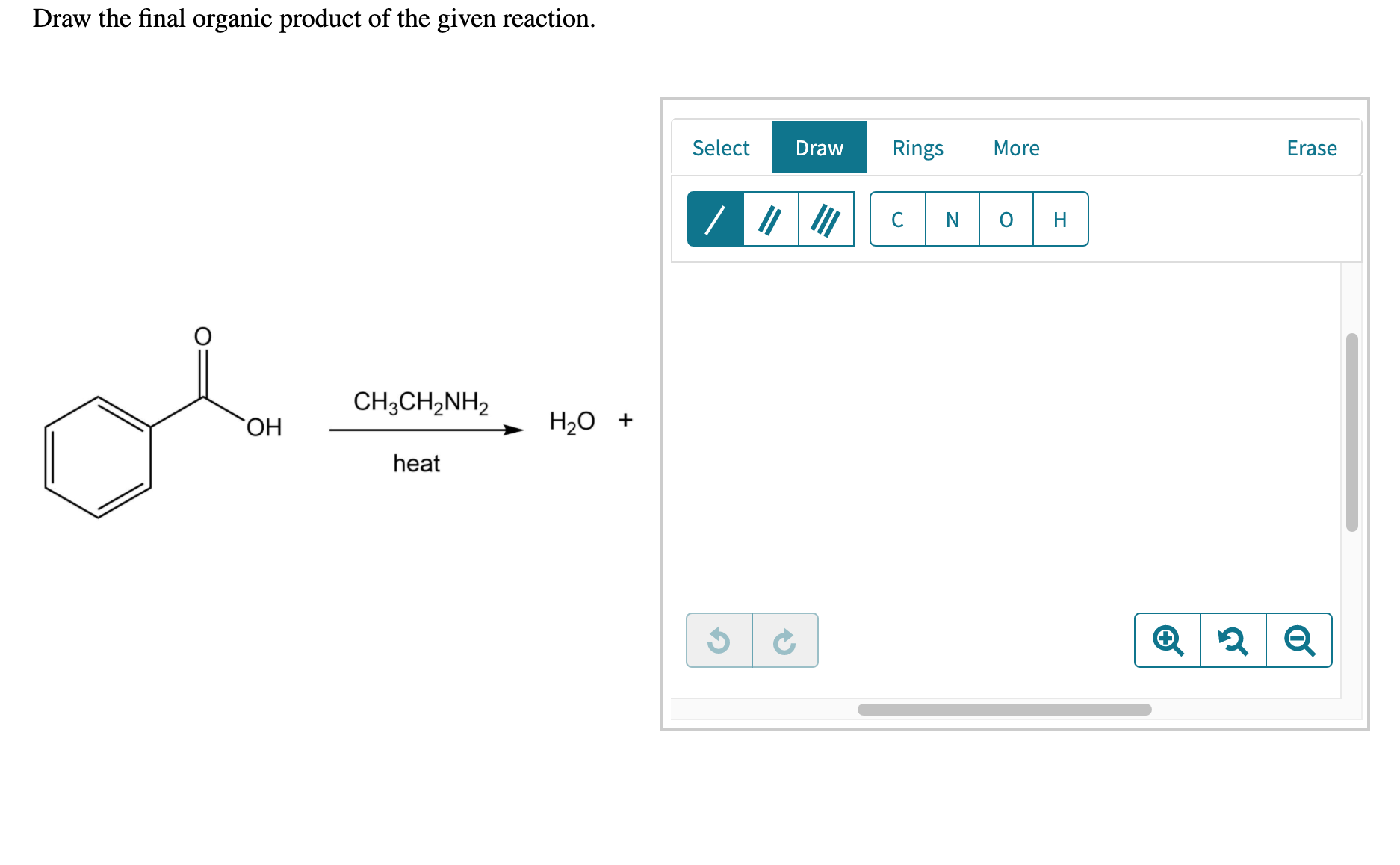
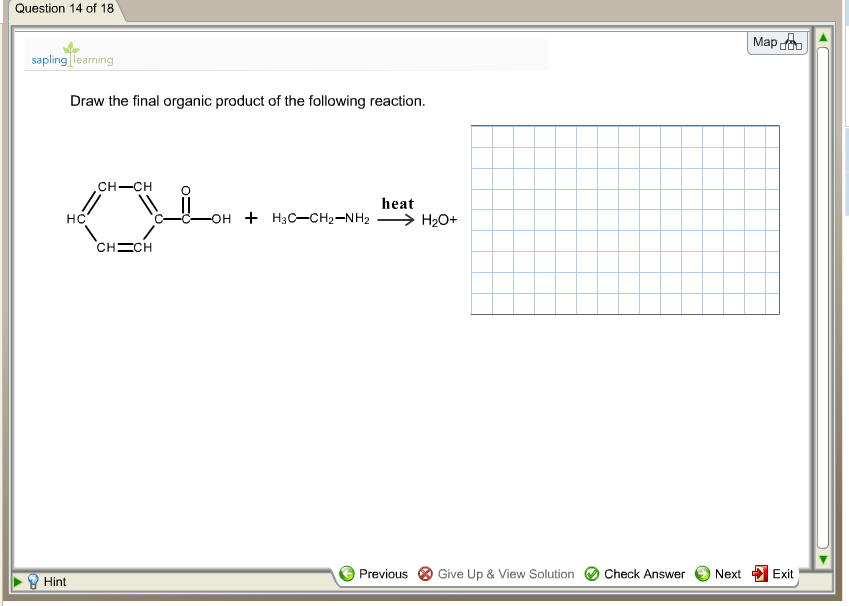
Web the resulting intermediate will lose a water molecule, forming a double bond between the nitrogen and the carbon. $\textbf {ch}_3\textbf {ch}_2\textbf {n}=\textbf {ch}$. In this lesson, you will learn about several general categories of organic reactions. The reaction can be represented as. Do not include lone pairs in your answer.
Solved Draw the final organic product of this series of
The reaction can be represented as. You do not have to consider stereochemistry. Draw the final organic product of this series of reactions. (1) name the organic product in (b)(i). Draw the fully displayed formula of the final organic product of this reaction.
Solved Draw the final organic product of the given reaction.
$\textbf {ch}_3\textbf {ch}_2\textbf {n}=\textbf {ch}$. Draw the final organic product of this series of reactions. The first reaction is between butyl bromide and hydroxide ion. Draw the final organic product of this series of reactions. • you do not have to explicitly draw h atoms.
Solved Draw the final product of this series of reactions.
This is an sn2 reaction where the hydroxide ion acts as a nucleophile and attacks the carbon atom attached to the bromine atom. Draw the final organic product of this series of reactions. H2cr04 you do not have to consider stereochemistry. When drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom,.
Solved Draw the final organic product of the following
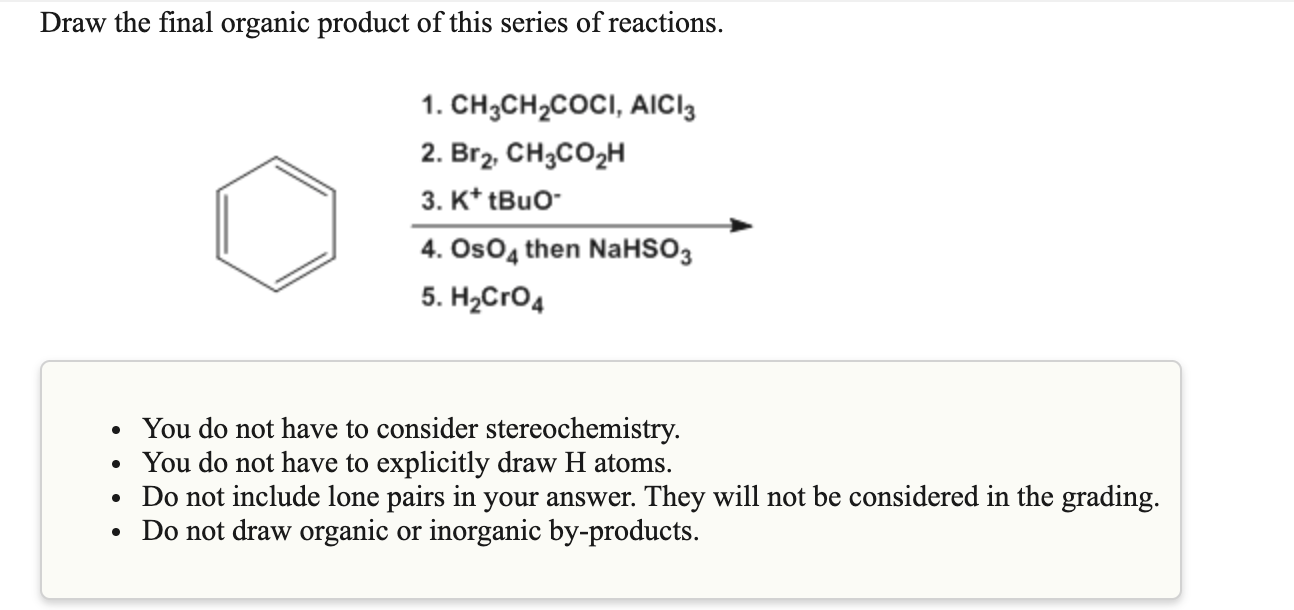
You do not have to explicitly draw h atoms. Web chemistry questions and answers. (1) name the organic product in (b)(i). Here’s some interesting results that experiments tell us. (ch3)2c(ch2ch3)ccch(ch3)2 2) hccch2ch2ch3 3) ch3ch=chch=chccch3 4) brch2ch2ccch2ch3.
Solved Draw The Final Organic Product Of This Series Of R...
You do not have to explicitly draw h atoms. Include hydrogen atoms in your structure. If the final product is a cis alkene, the immediate precursor might be an alkyne, which you could hydrogenate using the lindlar catalyst. They will not be considered in the grading. This creates a carbocation intermediate which reacts with the bromide ion to form the.
Draw the final organic product of this series of
The overall rate of a. You do not have to explicitly draw h atoms. This is an extremely common pattern for organic chemistry reactions. Here’s some interesting results that experiments tell us. Web the resulting intermediate will lose a water molecule, forming a double bond between the nitrogen and the carbon.
Solved Draw the final organic product of the given reaction.
Web in order for relatively stable organic molecules to react at a reasonable rate, they often must be modified with the use of highly reactive materials or in the presence of a catalyst. Web the sequence of individual steps, or elementary reactions, by which reactants are converted into products during the course of a reaction is called the reaction mechanism..
This Is An Sn2 Reaction Where The Hydroxide Ion Acts As A Nucleophile And Attacks The Carbon Atom Attached To The Bromine Atom.
A prime example can be seen in the metabolism of metabolites and. B− →−−mnox2 c b → m n o x 2 c. If no reaction occurs, draw the organic starting material. Not include lone pairs in your answer.
Web The Resulting Intermediate Will Lose A Water Molecule, Forming A Double Bond Between The Nitrogen And The Carbon.
Draw the final organic product of this series of reactions. Intermediate reactions are common in the biological world; You do not have to consider stereochemistry. H2cr04 you do not have to consider stereochemistry.
• • • Include The Amine From Which The Enamine Was Derived.
This problem has been solved! The reaction can be represented as. Include hydrogen atoms in your structure. This creates a carbocation intermediate which reacts with the bromide ion to form the final product.
Web Draw The Final Organic Product Of This Series Of Reactions.
Web alkene addition reaction with hbr occurs through the pi bond reacting as a nucleophile and abstracting a proton from the acid. • you do not have to explicitly draw h atoms. The bromine atom is then displaced, resulting in the formation of butanol. You do not have to consider stereochemistry.