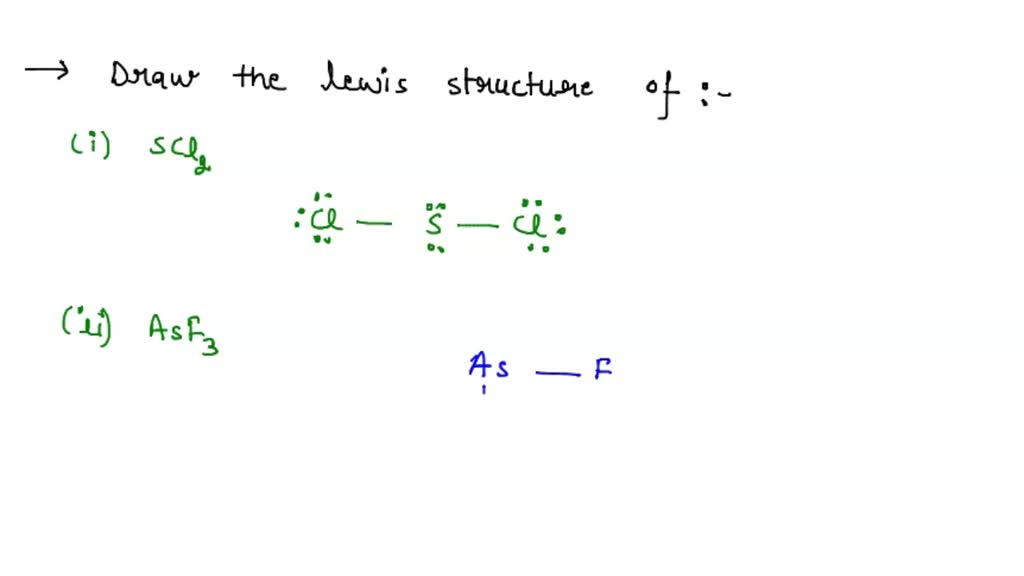Draw The Lewis Structure For Asf3
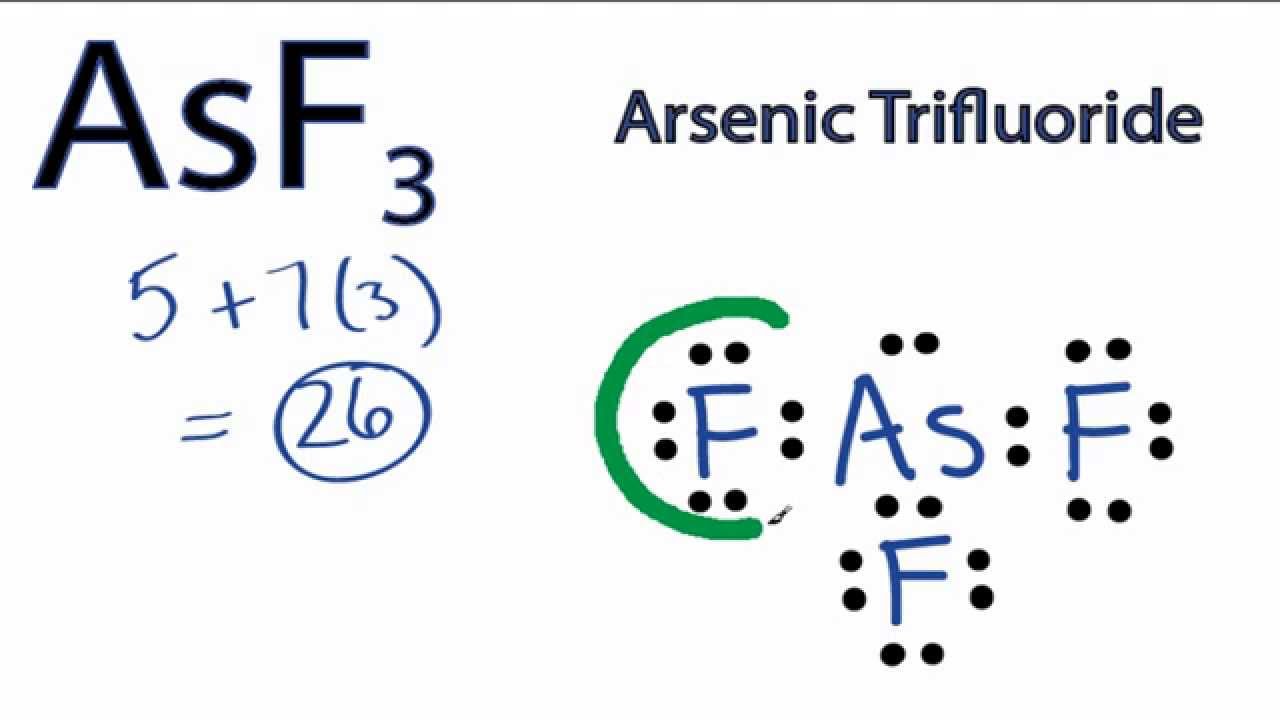
Draw The Lewis Structure For Asf3 - Be sure to show the formal charge on any. Each step is explained in detail in the remaining part of this tutorial. Web drawing the lewis structure for asf 3 (arsenic trihydride) the lewis structure for asf 3 is similar to ascl 3 structure. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). By using the following steps, you can easily draw the lewis structure of asf 3. #1 first draw a rough sketch. #2 mark lone pairs on the atoms. #3 calculate and mark formal charges. Web use these steps to correctly draw the asf 3 lewis structure: Draw the lewis structure for ch3+.
Web draw the lewis structure of asf 3. Web there are several steps to draw the lewis structure of the asf 3. Web drawing lewis structures for molecules with one central atom: Since they are in the same group on the periodic table. #2 mark lone pairs on the atoms. Each step is explained in detail in the remaining part of this tutorial. Web use these steps to correctly draw the asf 3 lewis structure: Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). Web arsenic trifluoride (asf3) has a central arsenic (as) atom with 5 valence electrons, bonded to three fluorine (f) atoms, each contributing 7 valence electrons. A) determine the number of lone pairs on the arsenic atom.
Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). #2 mark lone pairs on the atoms. Each step is explained in detail in the remaining part of this tutorial. Web drawing the lewis structure for asf 3 (arsenic trihydride) the lewis structure for asf 3 is similar to ascl 3 structure. Web the following procedure can be used to draw lewis structure for simple molecules. Find total number of electrons of the valance. #3 calculate and mark formal charges. Since they are in the same group on the periodic table. B) determine the number of shared pairs in the molecule. Using lewis dot symbols to.
AsF3 Lewis Structure,Geometry,Hybridization5 Steps (Solved)
Web use these steps to correctly draw the asf 3 lewis structure: Find total number of electrons of the valance. Web there are several steps to draw the lewis structure of the asf 3. Web draw the lewis structure of asf 3. #2 mark lone pairs on the atoms.
AsF3 Lewis Structure How to Draw the Lewis Structure for Arsenic
Web arsenic trifluoride (asf3) has a central arsenic (as) atom with 5 valence electrons, bonded to three fluorine (f) atoms, each contributing 7 valence electrons. Web the following procedure can be used to draw lewis structure for simple molecules. Find total number of electrons of the valance. Web drawing lewis structures for molecules with one central atom: Using lewis dot.
Arsenic Trifluoride Asf3 Chemspider
#1 first draw a rough sketch. B) determine the number of shared pairs in the molecule. Web draw the lewis structure of asf 3. To use lewis dot symbols to explain the stoichiometry of a compound. Using lewis dot symbols to.
AsF3 Lewis Structure (Arsenic Trifluoride) Lewis, Molecules, Electrons
B) determine the number of shared pairs in the molecule. Web the following procedure can be used to draw lewis structure for simple molecules. Be sure to show the formal charge on any. A) determine the number of lone pairs on the arsenic atom. Web draw the lewis structure of asf 3.
AsH3 Lewis Structure, Geometry, Hybridization, and Polarity
To use lewis dot symbols to explain the stoichiometry of a compound. Using lewis dot symbols to. Be sure to show the formal charge on any. #2 mark lone pairs on the atoms. Web arsenic trifluoride (asf3) has a central arsenic (as) atom with 5 valence electrons, bonded to three fluorine (f) atoms, each contributing 7 valence electrons.
AsF3 (Arsenic trifluoride) Molecular Geometry, Bond Angles YouTube
The following procedure will give you the correct lewis structure for any. Web drawing lewis structures for molecules with one central atom: Since they are in the same group on the periodic table. #2 mark lone pairs on the atoms. Draw the lewis structure for ch3+.
SOLVED 1) Draw the Lewis structure of A) SCl2 B) AsFr3 2) Using only
Web use these steps to correctly draw the asf 3 lewis structure: B) determine the number of shared pairs in the molecule. To use lewis dot symbols to explain the stoichiometry of a compound. Draw the lewis structure for ch3+. A) determine the number of lone pairs on the arsenic atom.
Draw the Lewis structure for AsF3 YouTube
Web arsenic trifluoride (asf3) has a central arsenic (as) atom with 5 valence electrons, bonded to three fluorine (f) atoms, each contributing 7 valence electrons. Using lewis dot symbols to. B) determine the number of shared pairs in the molecule. Web to draw the lewis structure of asf3, place the central arsenic (as) atom and connect it to three fluorine.
Leave a Comment Cancel Reply
Web the following procedure can be used to draw lewis structure for simple molecules. Since they are in the same group on the periodic table. Using lewis dot symbols to. B) determine the number of shared pairs in the molecule. Web arsenic trifluoride (asf3) has a central arsenic (as) atom with 5 valence electrons, bonded to three fluorine (f) atoms,.
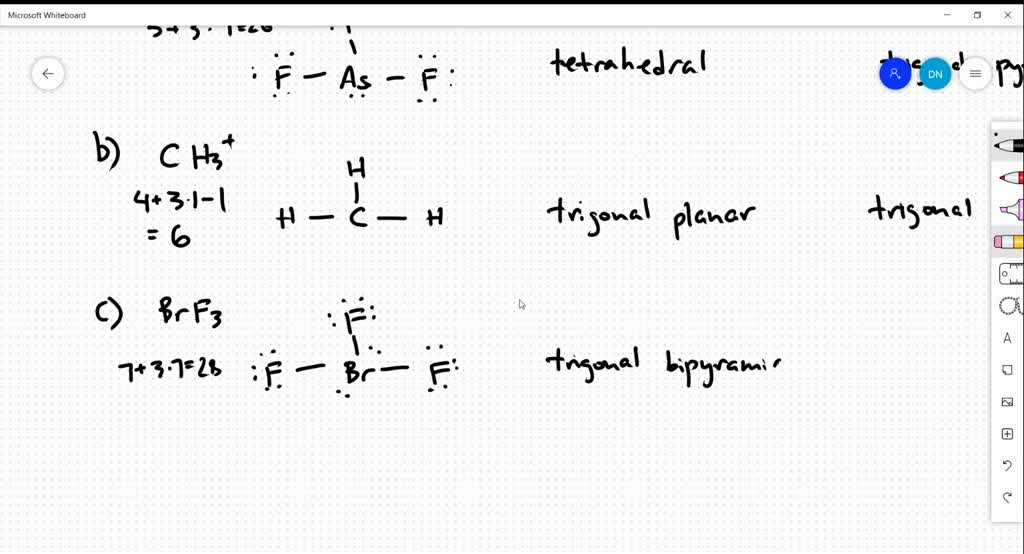
SOLVED Draw the Lewis structure for each of the following molecules or
#1 first draw a rough sketch. By using the following steps, you can easily draw the lewis structure of asf 3. A) determine the number of lone pairs on the arsenic atom. Each step is explained in detail in the remaining part of this tutorial. Using lewis dot symbols to.
#1 First Draw A Rough Sketch.
#3 calculate and mark formal charges. Web drawing the lewis structure for asf 3 (arsenic trihydride) the lewis structure for asf 3 is similar to ascl 3 structure. Web arsenic trifluoride (asf3) has a central arsenic (as) atom with 5 valence electrons, bonded to three fluorine (f) atoms, each contributing 7 valence electrons. Web to draw the lewis structure of asf3, place the central arsenic (as) atom and connect it to three fluorine (f) atoms using single bonds, and distribute the.
Web There Are Several Steps To Draw The Lewis Structure Of The Asf 3.
Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). Each step is explained in detail in the remaining part of this tutorial. Web the following procedure can be used to draw lewis structure for simple molecules. Using lewis dot symbols to.
The Following Procedure Will Give You The Correct Lewis Structure For Any.
By using the following steps, you can easily draw the lewis structure of asf 3. B) determine the number of shared pairs in the molecule. #2 mark lone pairs on the atoms. Web drawing lewis structures for molecules with one central atom:
Web Draw The Lewis Structure Of Asf 3.
Draw the lewis structure for ch3+. Be sure to show the formal charge on any. To use lewis dot symbols to explain the stoichiometry of a compound. Since they are in the same group on the periodic table.