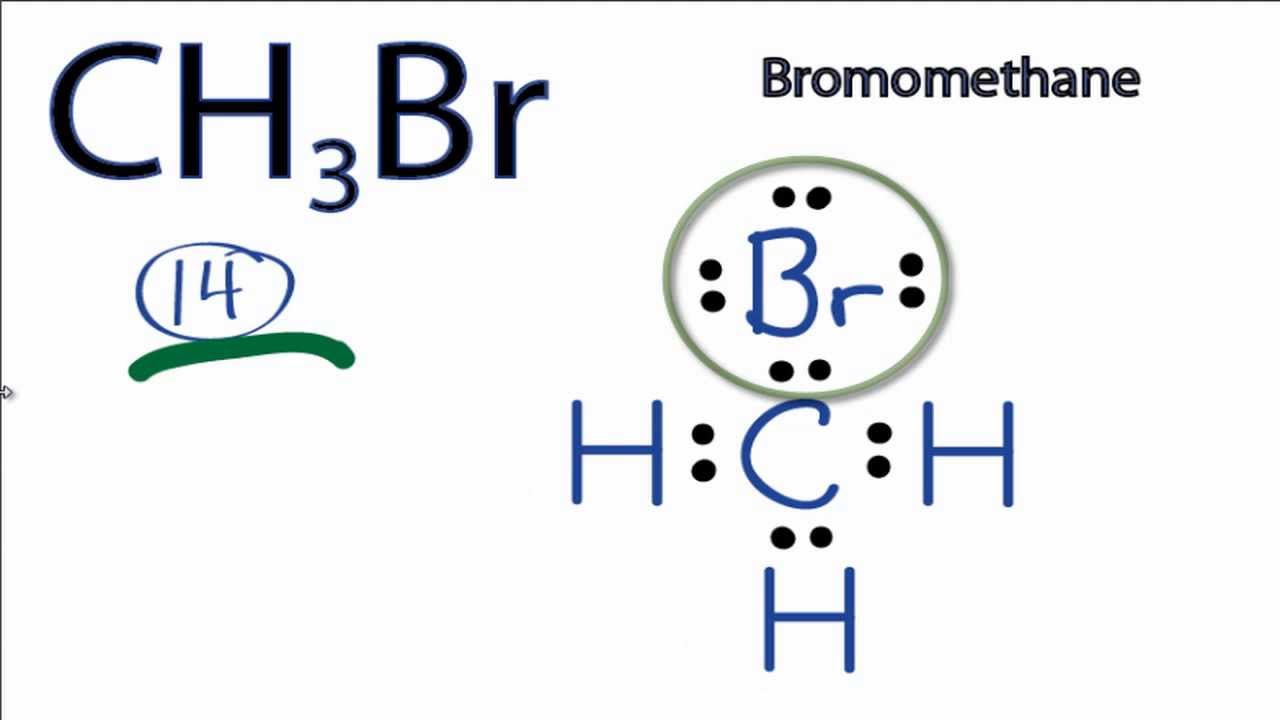
Draw The Lewis Structure For Ch3Br
Draw The Lewis Structure For Ch3Br - Count the total number of valence electrons for all the atoms in the molecule. You can change your selection by clicking or tapping on any part of the drawing, including the elements and bonds. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Hydrogen always goes on the outside, and since carbon is less electronegative than bromine, we'll put the carbon in the center and the bromine on top. Find the total valence electrons in ch3br molecule. Web to draw the ch3br lewis structure, follow these steps: For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. Use formal charges to identify the most reasonable lewis structure for a given molecule. (valence electrons are the electrons that are. Highlight the appropriate bond (s) by selecting the highlight function on the.
Web check me out: A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. You can draw them in any arrangement you like. First, lets find the how many valence electrons chlorate has: We have 14 valence electrons for the ch3br lewis structure. Compute formal charges for atoms in any lewis structure. For ch3br, there are a total of 14 valence electrons. Web chemistry questions and answers. Use formal charges to identify the most reasonable lewis structure for a given molecule. Here, the given molecule is ch3br.
In order to find the total valence electrons in a ch3br molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as bromine atom. Here’s the best way to solve it. Cl + + ☑ ho click and drag to start drawing a structure. Web steps of drawing ch3br lewis structure step 1: In order to draw the lewis structure of ch3br, first of all you have to find the total number of valence electrons present in the ch3br molecule. So the total number of valence electrons in ch3br is (4 + 3*1 + 7) = 14. You do not need to draw any of the side products of the reaction, only the substitution product. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Here’s the best way to solve it. Web chemistry questions and answers.
Draw The Lewis Structure Of Ch3br
Drawing styles vary from chemist to chemist, but most chemists draw covalent bonds as lines, and nonbonding electrons as dots. Web in the ch3br lewis structure, there are four single bonds around the carbon atom, with three hydrogen atoms and one bromine atom attached to it, and on the. Put two between atoms to form chemical bonds. You can change.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
Web to draw the ch3br lewis structure, follow these steps: Draw the lewis structure of ch3br. Bromine atom has 3 lone pairs. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. In order to draw the lewis structure of ch3br, first of all you have to find the total number of valence electrons.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
Lewis structures of molecular compounds is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Include lone pairs of electrons. Web for ch3br, there are a total of 14 valence electrons. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Bromine atom has 3 lone pairs.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
Bromine atom has 3 lone pairs. Use formal charges to identify the most reasonable lewis structure for a given molecule. Determine the central atom in the molecule. Draw the lewis structure of ch3br. Web draw the lewis structure of ch3br.
Draw The Lewis Structure Of Ch3Br at Drawing
100% (2 ratings) share share. For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. In the case of ch3br, carbon being a group 14 element, has 4 electrons in its valence shell. Calculate the total number of valence electrons. Web draw the lewis structure of ch3br.
[Solved] Draw the Lewis structure of bromoform (CH3Br). a. What is the
Web steps of drawing ch3br lewis structure step 1: Web just click or tap on the plus symbol to open the drawing tool. (valence electrons are the electrons that are. Find more chemistry widgets in wolfram|alpha. Identify the oxidation states of atoms in lewis structures.
Lewis Structure For Ch3br
Web to draw the ch3br lewis structure, follow these steps: Draw the lewis structure of ch3br. Web hello guys, welcome back to our channel, and in today's video, we share our quick and easy method to determine the lewis structure of the ch3br molecule. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency..
CH3Br Lewis Structure (Bromomethane) YouTube
Predict the major products of this organic reaction: For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. You do not need to draw any of the side products of the reaction, only the substitution product. Web draw lewis structures depicting the bonding in simple molecules. Hydrogen always goes on the outside, and since.
CH3Br Lewis Structure How to Draw the Lewis Structure for CH3Br
Web 6 steps to draw the lewis structure of ch3br step #1: Web to draw the ch3br lewis structure, follow these steps: Identify the oxidation states of atoms in lewis structures. In order to draw the lewis structure of ch3br, first of all you have to find the total number of valence electrons present in the ch3br molecule. Here’s the.
Chbr3 Lewis Structure
Here’s the best way to solve it. In order to find the total valence electrons in a ch3br molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as bromine atom. Web draw lewis structures depicting the bonding in simple molecules. Draw the lewis structure of ch3br. #4 calculate formal charge and.
100% (2 Ratings) Share Share.
• we will begin by calculating the total number of valence electrons in this molecule. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on. Cl + + ☑ ho click and drag to start drawing a structure. There are 26 valence electrons.
Web Check Me Out:
Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Web 6 steps to draw the lewis structure of ch3br step #1: You do not need to draw any of the side products of the reaction, only the substitution product. In order to draw the lewis structure of ch3br, first of all you have to find the total number of valence electrons present in the ch3br molecule.
Web Draw The Lewis Structure Ch3Br, What Is The Hybridization For The Catom?
First, lets find the how many valence electrons chlorate has: Calculate the total number of valence electrons. Web for ch3br, there are a total of 14 valence electrons. For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven.
In This Section, We Will Draw The Lewis Structure Of The Ch 3 Br Molecule Step By Step:
For the ch3br molecule (c is the central atom), draw the correct lewis structure and select all polar covalent bonds. Hydrogen always goes on the outside, and since carbon is less electronegative than bromine, we'll p. Web chemistry questions and answers. Hydrogen always goes on the outside, and since carbon is less electronegative than bromine, we'll put the carbon in the center and the bromine on top.