Draw The Lewis Structure For Ch3Cl
Draw The Lewis Structure For Ch3Cl - Chlorine has 7 valence electrons. Here, the given molecule is ch3cl. #2 mark lone pairs on the atoms. #1 first draw a rough sketch. Write lewis symbols for neutral atoms and ions. Web hey guys!in this video, we are going to learn about the lewis dot structure of chloromethane or methyl chloride having a chemical formula of ch3cl. #3 calculate and mark formal charges on the atoms, if required. How to draw lewis structure for ch3cl chloromethane (methylchloride) lewis structure: Draw the lewis structure for ch3cl. Select the intermolecular forces present between ch3cl molecules.
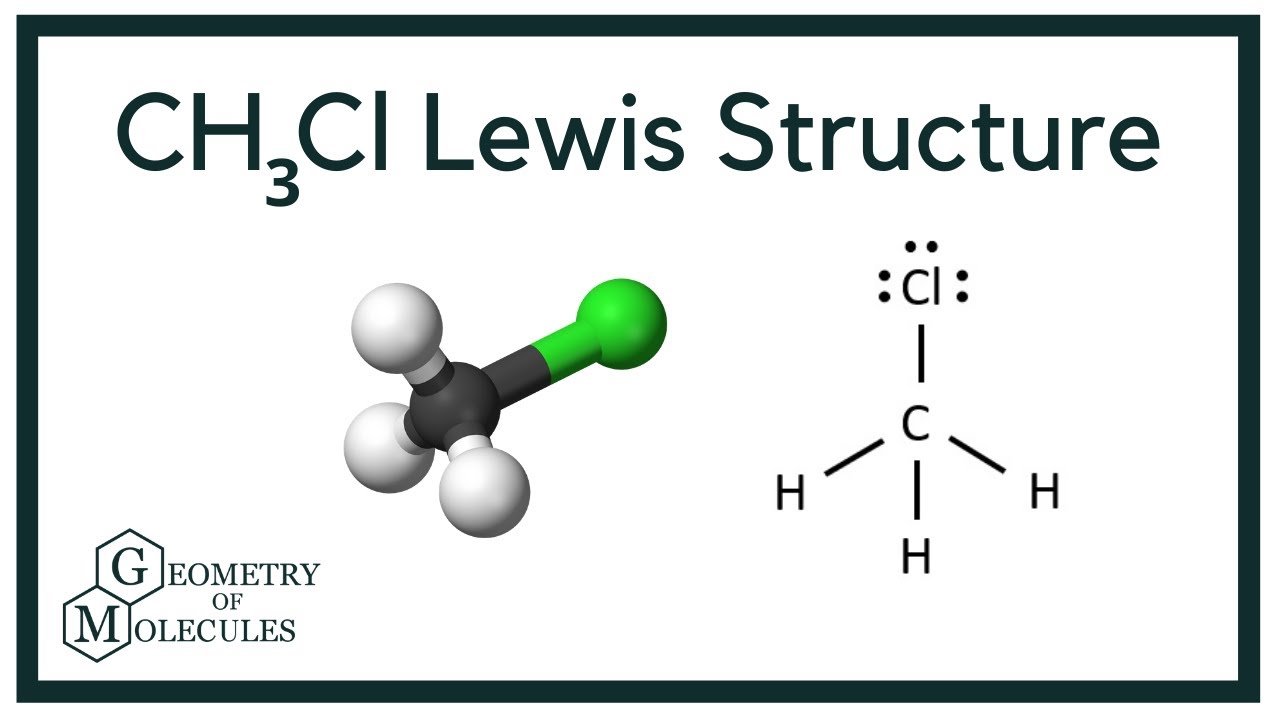
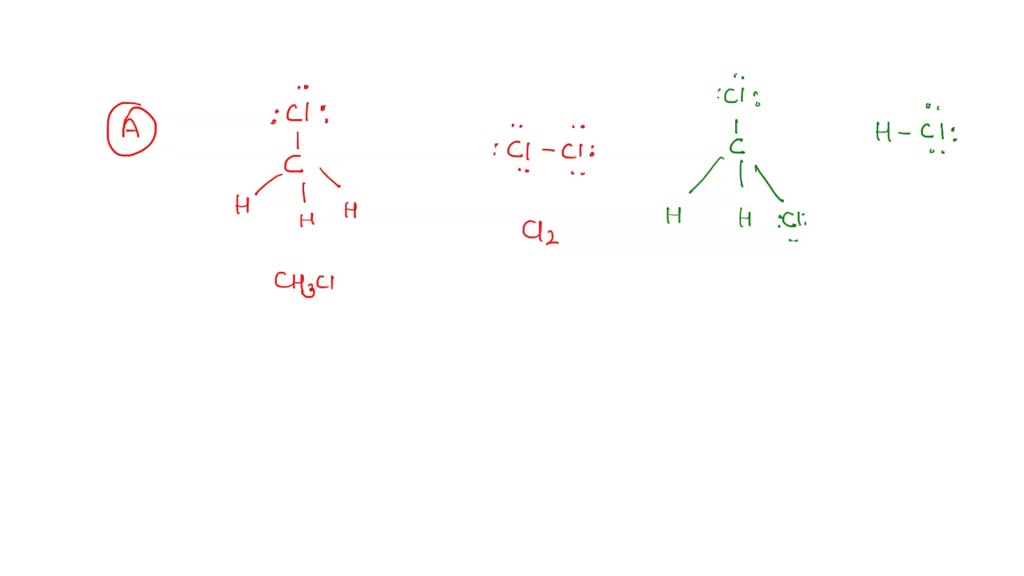
Chlorine has 7 valence electrons. The geometry around the central atom is ______. The lewis structure of ch3cl consists of a carbon (c) atom at the center which is bonded to three hydrogens (h) atoms and one atom of chlorine (cl). Web 6 steps to draw the lewis structure of ch3cl step #1: #1 first draw a rough sketch. There are 26 valence electrons. Calculate the total number of valence electrons. • how to draw lewis. By the end of this section, you will be able to: Web check me out:
Draw lewis structures depicting the bonding in simple molecules. #2 mark lone pairs on the atoms. Select the intermolecular forces present between ch3cl molecules. By the end of this section, you will be able to: Web this widget gets the lewis structure of chemical compounds. To use lewis dot symbols to explain the stoichiometry of a compound. #1 first draw a rough sketch. Web hey guys!in this video, we are going to learn about the lewis dot structure of chloromethane or methyl chloride having a chemical formula of ch3cl. 102k views 10 years ago. Carbon will go in the center.
Lewis Structure Ch3cl
102k views 10 years ago. Select the intermolecular forces present between ch3cl molecules. For the ch3cl structure use the periodic table. The geometry around the central atom is ______. Web define electronegativity and assess the polarity of covalent bonds.
What Is Ch3cl Lewis Structure?
Write lewis symbols for neutral atoms and ions. Next lets draw the basic framework of the molecule: Use these steps to correctly draw the ch 3 cl lewis structure: For the ch2cl2 structure use the. Web 6 steps to draw the lewis structure of ch3cl step #1:
Lewis Structure Ch3cl
The geometry around the central angle is ______. For the ch2cl2 structure use the. Hydrogens always go on the outside. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Chlorine has 7 valence electrons.
CHCl3Lewisstructure Chloroform Chloroformlewisstructure
There are 26 valence electrons. The geometry around the central angle is ______. First, determine the total number of valence electrons. Let’s discuss each step in more detail. Draw the lewis structure for ch3cl.
CH3Cl Lewis Structure + Geometry YouTube
Carbon will go in the center. Draw the lewis structure for chloromethane, ch3cl. The lewis structure of ch3cl consists of a carbon (c) atom at the center which is bonded to three hydrogens (h) atoms and one atom of chlorine (cl). To use lewis dot symbols to explain the stoichiometry of a compound. Web hey guys!in this video, we are.
CHCl3 Lewis Structure How to Draw the Lewis Structure for CHCl3 YouTube
To use lewis dot symbols to explain the stoichiometry of a compound. Use these steps to correctly draw the ch 3 cl lewis structure: Here, the given molecule is ch3cl. Web define electronegativity and assess the polarity of covalent bonds. Draw the lewis structure for ch2o.
CH3Cl Lewis Structure How to Draw the Lewis Structure for CH3Cl
Calculate the total number of valence electrons. Hydrogens always go on the outside. Write lewis symbols for neutral atoms and ions. Use these steps to correctly draw the ch 3 cl lewis structure: Hydrogen's in group 1 but we've got 3 hydrogens.
Lewis Structure Of Ch3cl
The lewis structure of ch3cl consists of a carbon (c) atom at the center which is bonded to three hydrogens (h) atoms and one atom of chlorine (cl). Web define electronegativity and assess the polarity of covalent bonds. Let’s discuss each step in more detail. We add them up, we get 14 total valence electrons. Draw the lewis structure for.
The Lewis Structure Of Ch3Cl Understanding The Molecular Geometry
Compute formal charges for atoms in any lewis structure. Web by using the following steps, you can easily draw the lewis structure of ch 3 cl: 2.3k views 3 years ago chemistry. #3 calculate and mark formal charges on the atoms, if required. The lewis structure of ch3cl consists of a carbon (c) atom at the center which is bonded.
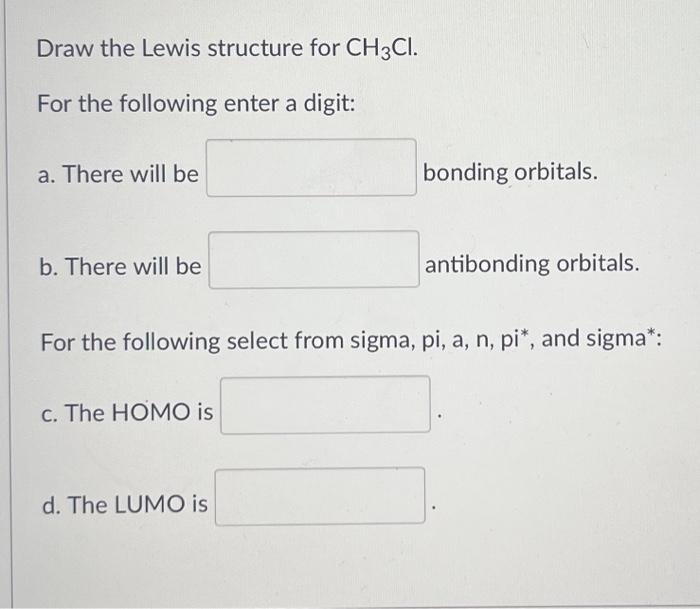
Solved Draw the Lewis structure for CH3Cl. For the following
Draw lewis structures depicting the bonding in simple molecules. The central atom is ______. • how to draw lewis. Draw lewis structures depicting the bonding in simple molecules. On the periodic table, carbon group 4 or 14, 4 valence electrons.
#2 Mark Lone Pairs On The Atoms.
2.3k views 3 years ago chemistry. 102k views 10 years ago. The lewis structure of ch3cl consists of a carbon (c) atom at the center which is bonded to three hydrogens (h) atoms and one atom of chlorine (cl). Using lewis dot symbols to describe covalent bonding.
This Problem Has Been Solved!
#3 calculate and mark formal charges on the atoms, if required. Next lets draw the basic framework of the molecule: Web by using the following steps, you can easily draw the lewis structure of ch 3 cl: Chlorine has 7 valence electrons.
To Use Lewis Dot Symbols To Explain The Stoichiometry Of A Compound.
First, lets find the how many valence electrons chlorate has: Hydrogen's in group 1 but we've got 3 hydrogens. Draw lewis structures depicting the bonding in simple molecules. #1 first draw a rough sketch.
Web Hey Guys!In This Video, We Are Going To Learn About The Lewis Dot Structure Of Chloromethane Or Methyl Chloride Having A Chemical Formula Of Ch3Cl.
The geometry around the central atom is ______. Hydrogens always go on the outside. The central atom is ______. First, determine the total number of valence electrons.