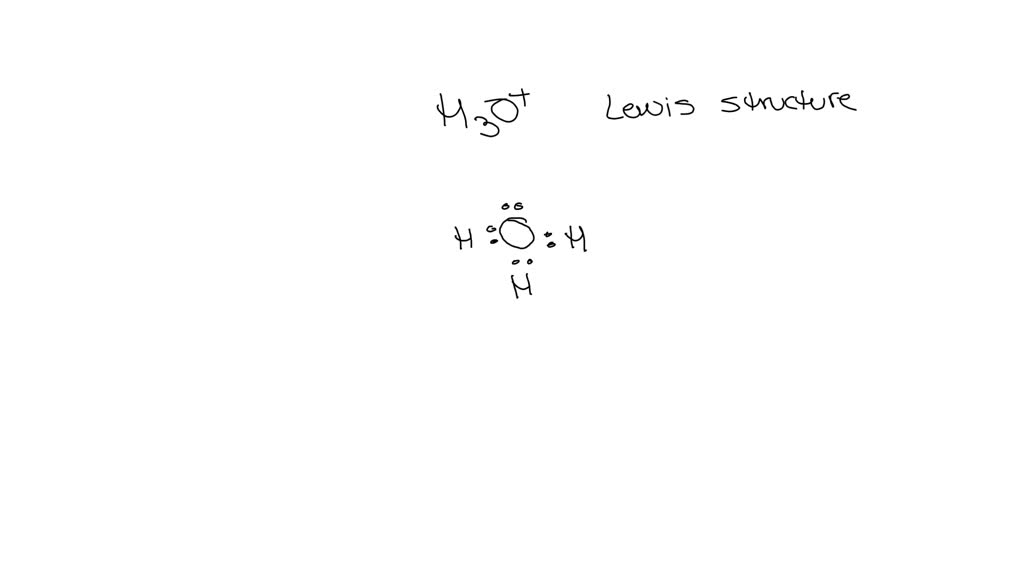
Draw The Lewis Structure For H3O
Draw The Lewis Structure For H3O - Web hydrogen atoms only need 2 valence electrons to have a full outer shell. Assume that bonding follows the octet rule. See the big list of lewis structures. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. To use lewis dot symbols to explain the stoichiometry of a compound. Web to properly draw the h 3 o + lewis structure, follow these steps: The ion acquires a positive charge after donating an electron. In order to draw the lewis structure of h3o+, first of all you have to find the total number of valence electrons present in the h3o+ ion. Web draw the lewis structure of hydronium (h3o+) and then determine its electron domain and molecular geometries. Drawing the lewis structure for h3o+.
Show all unshared pairs and the formal charges, if any. Drawing the lewis structure for h3o+. See the big list of lewis structures. This problem has been solved! Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Calculate the total number of valence electrons. The ion acquires a positive charge after donating an electron. I also go over hybridization, shape and bond angle. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Send feedback | visit wolfram|alpha.
H 3 o + lewis structure. Find more chemistry widgets in wolfram|alpha. Web the attached image below shows the lewis structure of hydronium ion; A) tetrahedral / planar b) tetrahedral / trigonal pyramidal c) planar / planar d) trigonal planar / linear. Drawing a lewis structure is pretty simple! Draw the lewis structure for h3o+. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Web draw the lewis structure for the (h3o+)ion. In order to find the total valence electrons in h3o+ ion, first of all you should know the valence electrons present in oxygen atom as well as hydrogen atom. Web drawing lewis structures for molecules with one central atom:
Draw a Lewis structure for H3O+ . Include all hydrogen atoms and show
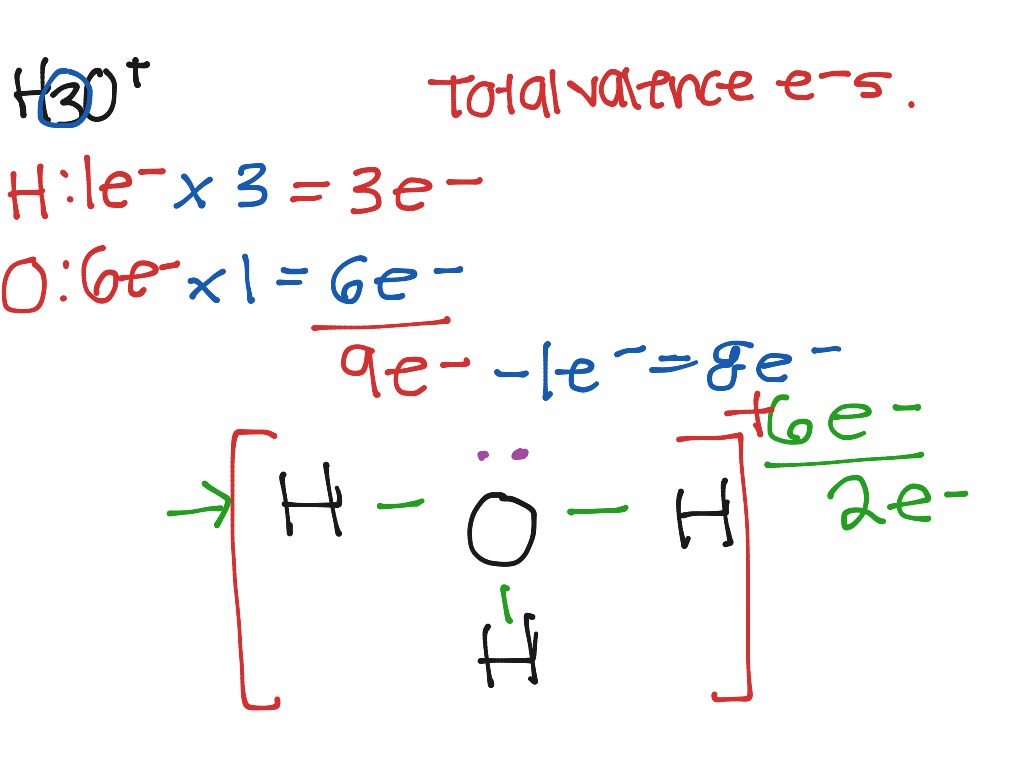
By using the following steps, you can easily draw the lewis structure of h 3 o +. Find more chemistry widgets in wolfram|alpha. For the h3o+ lewis structure we first count the valence electrons for the h3o+ molecule using the periodic table. Once we know how many valence electrons there are in h3o+ we can distribute them around the central.
SOLVED Draw the Lewis structure for H3O+. You must show adding up the
Each hydrogen atom has linked with oxygen atom. Draw a skeleton joining the atoms by single bonds. Show all unshared pairs and the formal charges, if any. Web draw the lewis structure of hydronium (h3o+) and then determine its electron domain and molecular geometries. #1 draw a rough sketch of the structure.
H3O+ Lewis Structure, Geometry, Hybridization, and MO Diagram
This problem has been solved! Web it consists of one hydrogen atom and three oxygen atoms. The ion acquires a positive charge after donating an electron. 8 + (2 × × 7) = 22 xef 6: #2 next, indicate lone pairs on the atoms.
Lewis Dot Structure for H3O^+ Science, Chemistry ShowMe
Find the total valence electrons in h3o+ ion. In order to find the total valence electrons in h3o+ ion, first of all you should know the valence electrons present in oxygen atom as well as hydrogen atom. Here, the given ion is h3o+ ion (hydronium ion). Show all unshared pairs and the formal charges, if any. A) tetrahedral / planar.
A stepbystep explanation of how to draw the H3O+ Lewis Structure
Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Added jun 9, 2014 by webtester in chemistry. To use lewis dot symbols to explain the stoichiometry of a compound. Web the attached image below shows the lewis structure of hydronium ion; Let’s break down each step in more detail.
H3O+ Lewis Structure Lewis Dot Structure for H3O+ Hydronium ion
This problem has been solved! Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Draw a skeleton joining the atoms by single bonds. Here, the given ion is h3o+ ion (hydronium ion). What is the hybridization on the o atom?
H3o+ Ion Lewis Structure Draw Easy
This problem has been solved! Drawing lewis structures for bf3, pf3 and brf3; Web it consists of one hydrogen atom and three oxygen atoms. What is the hybridization on the o atom? Drawing a lewis structure is pretty simple!
Lewis Dot Structure For H3o Science Chemistry Showme
Let’s break down each step in more detail. Drawing a lewis structure is pretty simple! 255 views 2 years ago. Web i quickly take you through how to draw the lewis structure of hydronium ion, h3o+. Web it consists of one hydrogen atom and three oxygen atoms.
How to Draw The Lewis Structure of The Hydronium Ion (H3O+) YouTube
This widget gets the lewis structure of chemical compounds. Calculate the number of valence electrons: Assume that bonding follows the octet rule. Web hydronium ion contains hydrogen and oxygen atoms. Draw the lewis structure for h3o+.
26+ H3O+ Molecular Geometry Shape Background GM
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. What is the hybridization on the o atom? Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Draw the lewis structure for h3o+. #2 next, indicate lone pairs on the atoms.
In This Case, We Can Condense The Last Few Steps, Since Not All Of Them Apply.
Drawing lewis structures for bf3, pf3 and brf3; Web drawing lewis structures for molecules with one central atom: Web to properly draw the h 3 o + lewis structure, follow these steps: Draw the lewis structure for h3o+.
In Order To Find The Total Valence Electrons In H3O+ Ion, First Of All You Should Know The Valence Electrons Present In Oxygen Atom As Well As Hydrogen Atom.
Only one lone pair exist on oxygen atom. Find more chemistry widgets in wolfram|alpha. Show all unshared pairs and the formal charges, if any. See the big list of lewis structures.
A Video Explanation Of How To Draw The Lewis Dot Structure For The Hydronium Ion, Along With.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. #1 draw a rough sketch of the structure. Web i quickly take you through how to draw the lewis structure of hydronium ion, h3o+. This problem has been solved!
This Problem Has Been Solved!
Web draw the lewis structure of hydronium (h3o+) and then determine its electron domain and molecular geometries. 8 + (6 × × 7) = 50; Find the total valence electrons in h3o+ ion. What is the hybridization on the o atom?