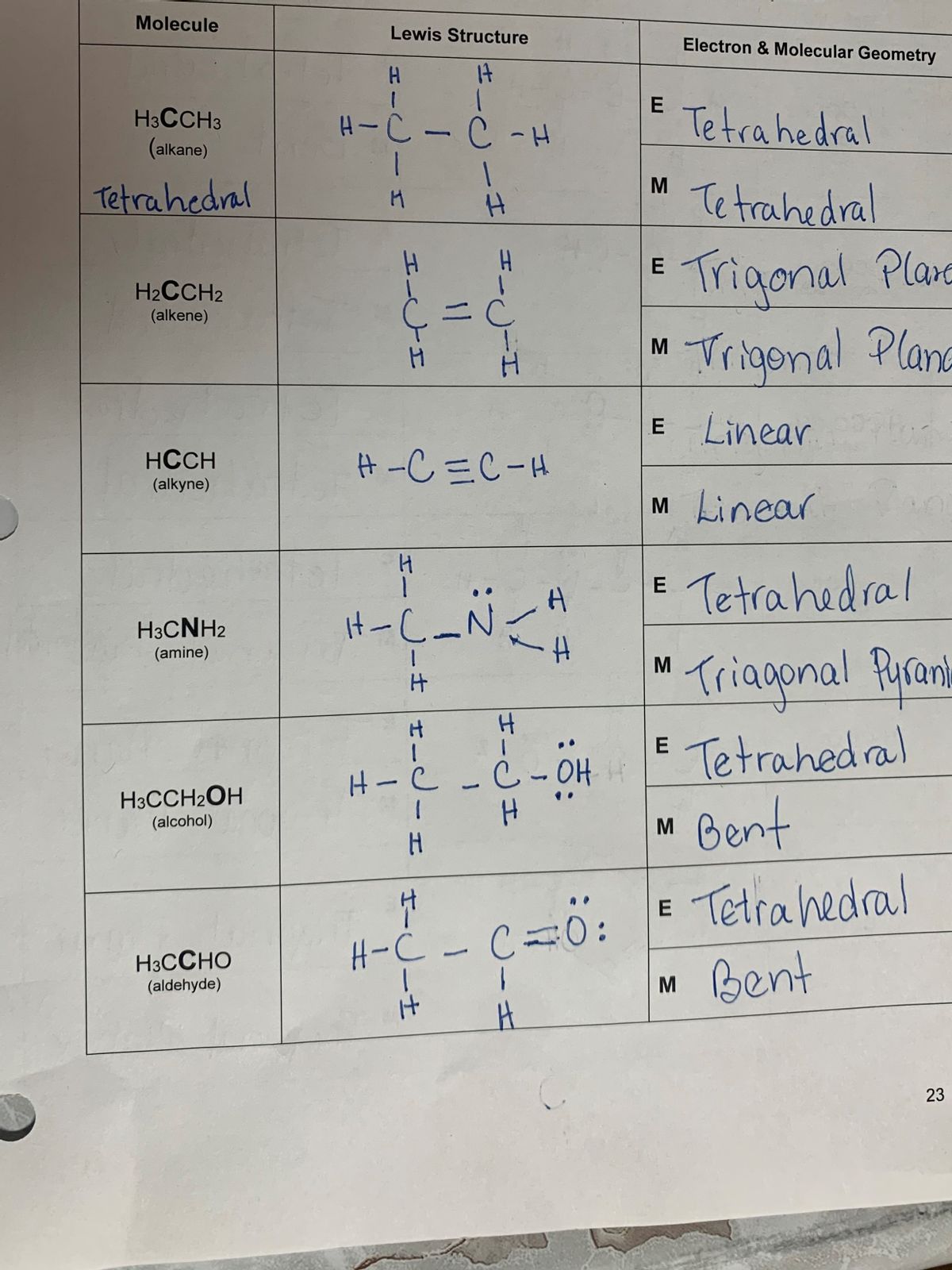
Draw The Lewis Structure For Hcch
Draw The Lewis Structure For Hcch - Web drawing lewis structures for molecules with one central atom: Start by determining the total number of valence electrons in the molecule. Additionally, we will look at lewis structures for molecules that violate the octet rule. Hcch lewis structure involves 2 atoms namely carbon and hydrogen. No terminal atoms capable of accepting electrons; Draw the lewis structure for cocl2. So according to hcch lewis structure formula c2h2 there are a total 4×2 + 2×1 = 10 valence electrons. To do this, we add up the valence electrons of each atom: Remember that h is never a central atom: Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions.
Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. No terminal atoms capable of accepting electrons Web drawing the lewis structure for hcch. In this case, h represents hydrogen, which has one valence electron, and c represents carbon, which has four valence electrons. Remember that h is never a central atom: By the end of this section, you will be able to: Start by determining the total number of valence electrons in the molecule. You'll get a detailed solution from a subject matter expert. Valence electrons in c = 4 electrons. Include all hydrogen atoms and all lone pairs of electrons reset ?
Web draw a skeleton and connect the atoms with single bonds. Web drawing lewis structures for molecules with one central atom: Remember that h is never a central atom: Carbon has 4 valence electrons and hydrogen has 1 valence electron. By the end of this section, you will be able to: Start by determining the total number of valence electrons in the molecule. Remember that h is never a central atom: Thus far, we have discussed the various types of bonds that form between atoms and/or ions. For the hcch lewis structure you'll need to form a triple bond between the two carbon atoms. Write lewis symbols for neutral atoms and ions.
Draw the Lewis structure for HCCH.Draw the molecule b… SolvedLib
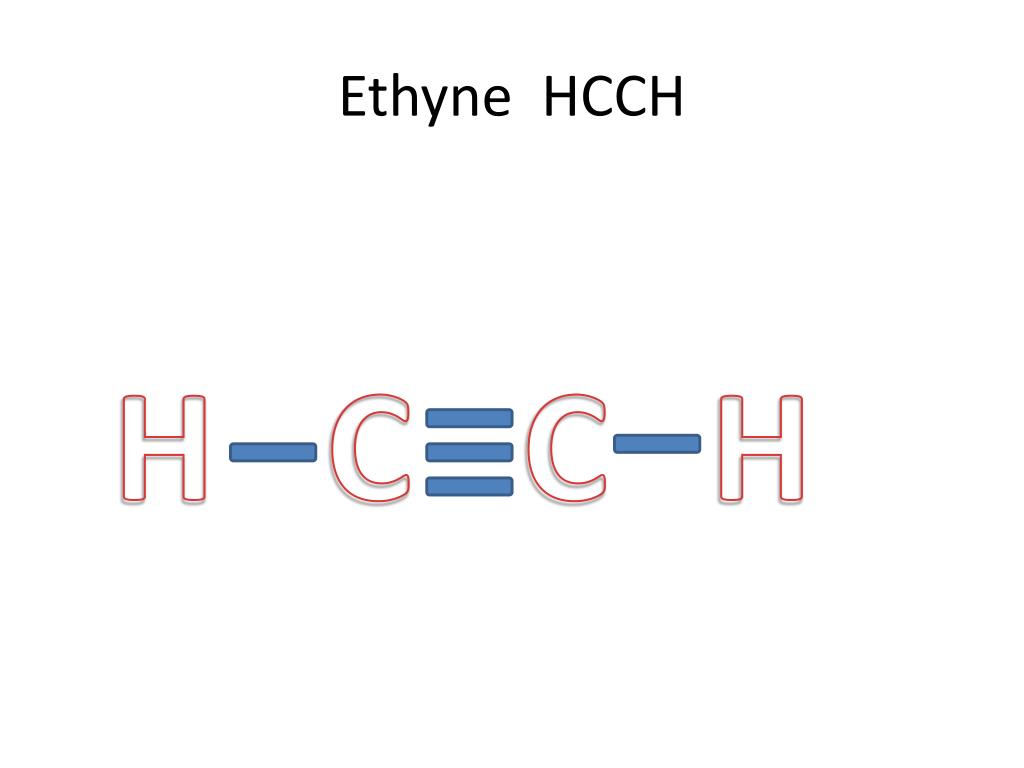
By the end of this section, you will be able to: Ethyne is also called acetylene. Web the final lewis structure for hcch (ethyne) is: Carbon has 4 valence electrons and hydrogen has 1 valence electron. Draw the lewis structure for cocl2.
PPT Drawing Lewis Structures PowerPoint Presentation, free download
For the hcch lewis structure you'll need to form a triple bond between the two carbon atoms. 100% (11 ratings) share share. Here’s the best way to solve it. To do this, we add up the valence electrons of each atom: We’ll first define what a lewis structure is and how lewis structures are made considering the octet rule.
Draw the Lewis structure for HCCH (ethyne) Draw the … SolvedLib
For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Valence electrons in c = 4 electrons. No terminal atoms capable of accepting electrons Web to draw the lewis structure for hcch ( acetylene ), follow the below steps: H h \ / c≡c | c.
SOLVED [SHOW WORK] Draw the lewis structure for HCCH molecule and
For the hcch lewis structure you'll need to form a triple bond between the two carbon atoms. Web draw lewis structures depicting the bonding in simple molecules. To recognize molecules that are likely to have multiple covalent bonds. We have discussed the various types of bonds that form between atoms. By the end of this section, you will be able.
HCCH Lewis structure ,Valence Electrons, Formal Charge
We have discussed the various types of bonds that form between atoms. Six electrons placed on n h 3 cch 3: Hcch lewis structure involves 2 atoms namely carbon and hydrogen. In this case, h represents hydrogen, which has one valence electron, and c represents carbon, which has four valence electrons. Draw lewis structures depicting the bonding in simple molecules.
Part A Draw the Lewis structure for HCCH (ethyne). Draw the molecule by
Remember that h is never a central atom: In this case, h represents hydrogen, which has one valence electron, and c represents carbon, which has four valence electrons. Write lewis symbols for neutral atoms and ions. Draw the molecule by placing atoms on the grid and connecting them with bonds. Where needed, distribute electrons to the terminal atoms:
Hcch Lewis Dot Structure Draw Easy
Web to draw lewis structures. Draw the lewis structure of hcch and then determine its electron domain and molecular geometries. So according to hcch lewis structure formula c2h2 there are a total 4×2 + 2×1 = 10 valence electrons. No terminal atoms capable of accepting electrons nh 3: For the hcch lewis structure you'll need to form a triple bond.
HCCH Lewis Structure How to Draw the Lewis Structure for the HCCH
Six electrons placed on n h 3 cch 3: So according to hcch lewis structure formula c2h2 there are a total 4×2 + 2×1 = 10 valence electrons. To draw the lewis structure for hcch (ethyne), we need to follow these steps: H h \ / c≡c | c / \ h h. Include all hydrogen atoms and all lone.
Part A Draw the Lewis structure for HCCH (ethyne). Draw the molecule by
No terminal atoms capable of accepting electrons; Write lewis symbols for neutral atoms and ions. Include all hydrogen atoms and all lone pairs of electrons reset ? Web to draw the lewis structure for hcch ( acetylene ), follow the below steps: 6.1 lewis symbols and structures.
Hcch Lewis Structure Molecular Geometry Draw Easy
Count the total number of valence electrons. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Draw lewis structures depicting the bonding in simple molecules. These bonds involve the sharing or transfer of valence shell electrons between atoms. Valence electrons in c = 4 electrons.
By The End Of This Section, You Will Be Able To:
Web draw a skeleton and connect the atoms with single bonds. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. (note that c is the central atom.) draw the molecule by placing atoms on the canvas and connecting them with bonds. This section explores lewis structures and how to draw them.
Thus Far In This Chapter, We Have Discussed The Various Types Of Bonds That Form Between Atoms And/Or Ions.
Web the final lewis structure for hcch (ethyne) is: Where needed, distribute electrons to the terminal atoms: Web draw lewis structures depicting the bonding in simple molecules. No terminal atoms capable of accepting electrons;
Remember That H Is Never A Central Atom:
Hcch (ethyne) can also be written as c 2 h 2. Web to draw the lewis structure for hcch ( acetylene ), follow the below steps: H h \ / c≡c | c / \ h h. Remember that h is never a central atom:
Draw The Lewis Structure Of Hcch And Then Determine Its Electron Domain And Molecular Geometries.
Additionally, we will look at lewis structures for molecules that violate the octet rule. Web drawing lewis structures for molecules with one central atom: Find out the total number of valence electrons of all atoms.valence electrons in h = 1 electron. Web to draw lewis structures.
![SOLVED [SHOW WORK] Draw the lewis structure for HCCH molecule and](https://cdn.numerade.com/project-universal/previews/293abd0a-096b-4553-a305-07c0382285dc_large.jpg)