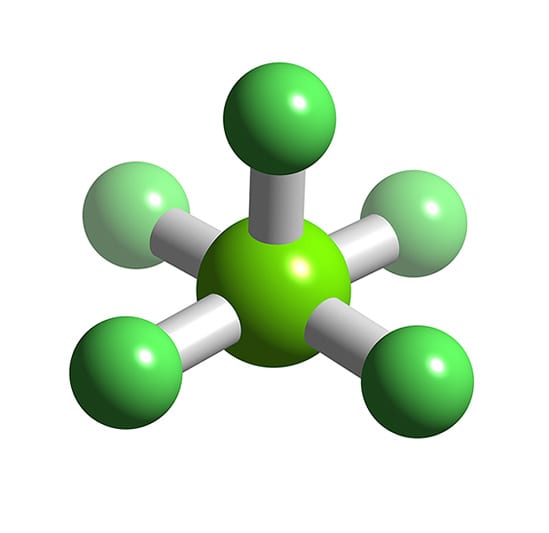
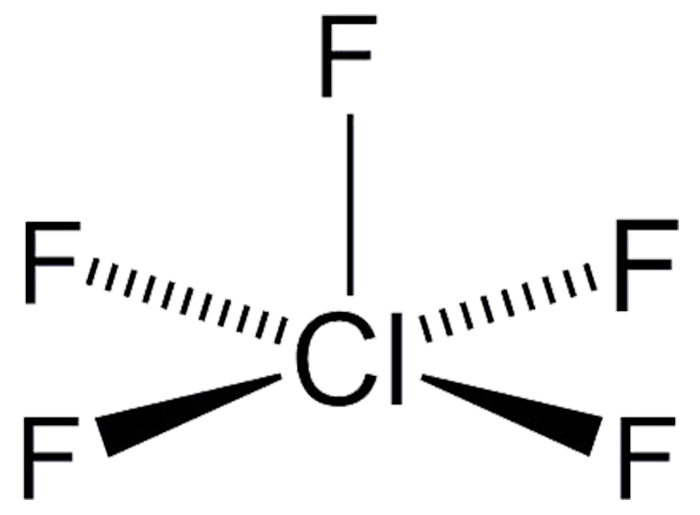
Draw The Lewis Structure For The Chlorine Pentafluoride Molecule
Draw The Lewis Structure For The Chlorine Pentafluoride Molecule - Draw the lewis electron structure of the molecule or polyatomic ion. You'll get a detailed solution from a subject matter expert that helps. Smith in 1963 by the reaction of clf 3 with f 2 under heat and pressure. Web the correct order of increasing acidic strength of the oxoacids of chlorine is: Here’s how you can easily draw the clf 5 lewis structure step by step: Web here’s how to approach this question. #2 mention lone pairs on the atoms. To use lewis dot symbols to explain the stoichiometry of a compound. Web draw the lewis structure of chlorine pentafluoride. Web the gas chlorine pentafluoride (clf 5) was first prepared by d.
Web the gas chlorine pentafluoride (clf 5) was first prepared by d. Count the total number of valence electrons for chlorine and fluorine to use in the lewis structure. Ć с so c e х $ explanation check ar c 30 f3 q 76 f1 f2 f5 @ #3 w# $ 4. Web the molecule is a planar dimer, with each iodine atom surrounded by four chlorine atoms. Web draw the lewis structure for the chlorine pentafluoride (clf5) molecule. Identify both its electron pair and molecular geometries. Web learn about the formula and structure of chlorine pentafluoride, a molecular compound with five fluorine atoms bonded to a central chlorine atom. Cl2 acts as a bleaching agent. Send feedback | visit wolfram|alpha. You'll get a detailed solution from a subject matter expert that helps.
Web interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university courses and advanced school chemistry. Ć с so c e х $ explanation check ar c 30 f3 q 76 f1 f2 f5 @ #3 w# $ 4. Web chlorine pentafluoride is an interhalogen compound with formula clf 5. Draw the lewis structure for the chlorine pentafluoride (cif:) molecule. Web here’s how to approach this question. The lewis structure indicates that each cl atom has three pairs of electrons. Draw the lewis electron structure of the molecule or polyatomic ion. Web draw the lewis electron structure of the molecule or polyatomic ion. Determine the electron group arrangement. Here’s how you can easily draw the clf 5 lewis structure step by step:
ClF5 Chlorine pentafluoride
#2 mention lone pairs on the atoms. #1 draw a rough skeleton structure. Web interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university courses and advanced school chemistry. Web chlorine pentafluoride is an interhalogen compound with formula clf 5. Web learn about the formula and structure of chlorine pentafluoride, a molecular compound.
Hybridization of ClF5 (Chlorine pentafluoride) YouTube
This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. Web draw the lewis structure for the chlorine pentafluoride (clf5) molecule. Web draw the lewis electron structure of the molecule or polyatomic ion. This widget gets the lewis structure of chemical compounds. To use lewis dot symbols to explain the stoichiometry of a compound.
[Solved] Draw the Lewis structure of chlorine pentafluoride. Identify
Web interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university courses and advanced school chemistry. Web for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: #1 draw a rough skeleton structure. If an overall dipole moment is present, draw the arrow.please. This problem has been.
Chlorine Pentafluoride Lewis / Chlorine pentafluoride is a square
This problem has been solved! #2 mention lone pairs on the atoms. Cl2 acts as a bleaching agent. Web draw the lewis structure of chlorine pentafluoride. To use lewis dot symbols to explain the stoichiometry of a compound.
ClF5 (Chlorine pentafluoride) Molecular Geometry, Bond Angles YouTube
Identify both its electron pair and molecular geometries. You'll get a detailed solution from a subject matter expert that helps. Web here’s how to approach this question. Ć с so c e х $ explanation check ar c 30 f3 q 76 f1 f2 f5 @ #3 w# $ 4. This colourless gas is a strong oxidant that was once.
Chlorine Pentafluoride Lewis Dot Diagram First draw the lewis dot
Tincture of iodine is _____. The set with the correct order of acidity is:. Draw the lewis structure for the chlorine pentafluoride (cif:) molecule. Web draw the lewis structure for the chlorine pentafluoride (clf5) molecule. Web here’s how to approach this question.
ClF5 Lewis Structure How to Draw the Lewis Structure for ClF5
If an overall dipole moment is present, draw the arrow.please. Web learn about the formula and structure of chlorine pentafluoride, a molecular compound with five fluorine atoms bonded to a central chlorine atom. Cl2 acts as a bleaching agent. Ć с so c e х $ explanation check ar c 30 f3 q 76 f1 f2 f5 @ #3 w#.
ClF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Web the correct order of increasing acidic strength of the oxoacids of chlorine is: Count the total number of valence electrons for chlorine and fluorine to use in the lewis structure. Added jun 9, 2014 by webtester in chemistry. Here’s how you can easily draw the clf 5 lewis structure step by step: Web draw the lewis electron structure of.
ClF5 Lewis Structure (Chlorine Pentafluoride) YouTube
Web a video explanation of how to draw the lewis dot structure for chlorine pentafluoride, along with information about the compound including formal charges, po. Web the correct order of increasing acidic strength of the oxoacids of chlorine is: This problem has been solved! Web for example, when two chlorine atoms form a chlorine molecule, they share one pair of.
Chlorine pentafluoride American Chemical Society
Web learn about the formula and structure of chlorine pentafluoride, a molecular compound with five fluorine atoms bonded to a central chlorine atom. Web for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Draw the lewis structure for the chlorine pentafluoride (cif:) molecule. Web draw the lewis electron structure of the molecule or.
Web Chlorine Pentafluoride Is An Interhalogen Compound With Formula Clf 5.
This problem has been solved! Cl2 acts as a bleaching agent. Determine the electron group arrangement. Web draw the lewis electron structure of the molecule or polyatomic ion.
This Widget Gets The Lewis Structure Of Chemical Compounds.
Count the total number of valence electrons for chlorine and fluorine to use in the lewis structure. If an overall dipole moment is present, draw the arrow.please. The set with the correct order of acidity is:. To use lewis dot symbols to explain the stoichiometry of a compound.
Explore The 3D Model And.
Web here’s how to approach this question. #1 draw a rough skeleton structure. Web the correct order of increasing acidic strength of the oxoacids of chlorine is: Tincture of iodine is _____.
Web Learn About The Formula And Structure Of Chlorine Pentafluoride, A Molecular Compound With Five Fluorine Atoms Bonded To A Central Chlorine Atom.
Web draw the lewis structure of chlorine pentafluoride. Determine the electron group arrangement around the central atom that minimizes. You'll get a detailed solution from a subject matter expert that helps. Web interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university courses and advanced school chemistry.