Draw The Lewis Structure For The Formaldehyde Ch2O Molecule
Draw The Lewis Structure For The Formaldehyde Ch2O Molecule - Be sure to include all resonance structures that satisfy the octet rule. Lewis structure is a pictorial representation of the atoms in the molecules, their bonds, and lone pairs of electrons. (1) find the number of valence electrons in the molecule. There are 2 steps to solve this one. Web lewis structure of ch2o. Be sure to include all resonance structures that satisfy the octet rule. Be sure to include all resonance structures that satisfy the octet rule. Phenol formaldehyde is an example of compound, which shows the chemical properties of formaldehyde. Draw the lewis structure and answer the following question. How to draw lewis structure of ch 2 o.
Drawing the ch2o lewis structure involves several steps: Polarity of ch 2 o; Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web i quickly take you through how to draw the lewis structure of ch2o (methanal or formaldehyde). 4 (c) + 2 (h) + 6 (o) = 12. Start with the valence electrons. Be sure to include all resonance structures that satisfy the octet rule. Web formaldehyde lewis structure. But we have two hydrogens, so let's multiply that by 2. How to draw lewis structure of ch 2 o.
So, the total number of valence electrons in ch2o is: Be sure to include all resonance structures that satisfy the octet rule. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw the lewis structure for the formaldehyde (ch2o) molecule. In this structure, the carbon atom is the central atom, bonded to two hydrogen atoms and one oxygen atom. For the ch2o structure use the periodic table to find the total number of valence electrons for. Hydrogen, in group 1, has 1 and oxygen, in group 6 or 16, has 6; Web lewis structure of ch2o. How to draw lewis structure of ch 2 o. Draw the lewis structure and answer the following question.
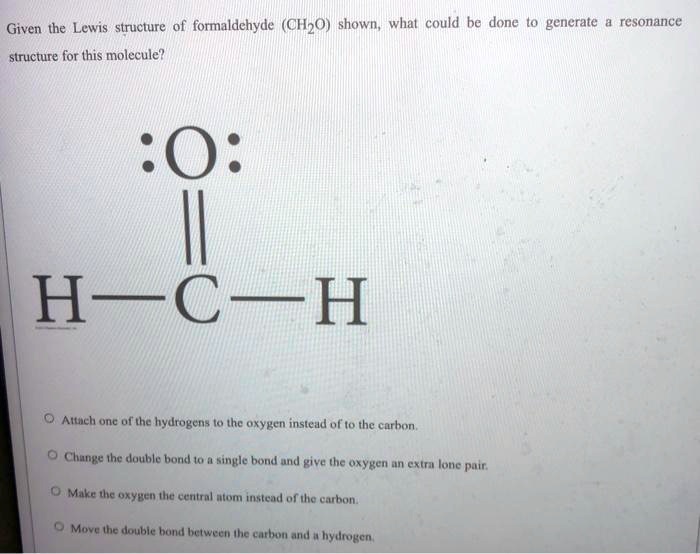
SOLVED Given the Lewis structure of formaldehyde (CH2O) shown; whal
Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. 4 (c) + 2 (h) + 6 (o) = 12. Draw the lewis structure and answer the following question. Be sure to include all resonance structures that satisfy the octet rule. Be sure to include all resonance structures.
Lewis Structure of CH2O (formaldehyde) YouTube
Web let's do the lewis structure for ch2o, methanal or formaldehyde. Draw the lewis structure and answer the following question. This problem has been solved! So, the total number of valence electrons in ch2o is: Web the lewis structure for ammonia (nh₃) shown below is incorrect.
How to Draw the Lewis Dot Structure for CH2O (Formaldehyde) YouTube
Web draw the lewis structure for the formaldehyde (ch2o) molecule. There is a double bond between oxygen and carbon atom. Draw out the lewis structure and use it to determine the electron domain geometry (edg) and molecular geometry (mg). Carbon (c) is the least electronegative. (1) find the number of valence electrons in the molecule.
How to Draw the Lewis Dot Structure for CH2O Formaldehyde YouTube
Start with the valence electrons. Hybridization of ch 2 o; Carbon (c) is the least electronegative. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. (1) find the number of valence electrons in the molecule.
Lewis Structure of Formaldehyde, CH2O YouTube
This problem has been solved! Draw a lewis structure for each of the following molecules or ions: Draw the lewis structure for the formaldehyde (ch2o) molecule. (3) distribute the remaining electrons throughout the molecule, keeping in mind the duet and octet rules. Draw the lewis structure for the formaldehyde (ch2o) molecule.
CH2O Lewis Structure, Molecular Geometry, and Hybridization
The number of valence electrons for carbon is 4, oxygen is 6, and hydrogen is 1. The lewis structure of the compound is only responsible for identifying the participation of electrons in making the molecular structure. There are 3 steps to solve this one. Draw the lewis structure for the formaldehyde (ch2o) molecule. Phenol formaldehyde is an example of compound,.
Formaldehyde Ch2o Molecule Chemical Structure stockillustratie
This problem has been solved! The lewis structure of ch2o is drawn as: Draw a lewis structure for each of the following molecules or ions: There are 2 steps to solve this one. Web the lewis structure for formaldehyde (ch2o) can be represented as follows:
CH2O Molecular Geometry,Shape and Bond Angles (Formaldehyde
*carbon can form a maximum of 4 bonds by sharing its four va. 4 (c) + 2 (h) + 6 (o) = 12. Web how to draw lewis structure for ch 2 o; Select an answer and submit. Web formaldehyde lewis structure.
CH2O Lewis Structure, Molecular Geometry, and Hybridization
Determine the total number of valence electrons: Carbon atom is located as the center atom in hcho. Web the compound formaldehyde has the molecular formula ch 2 o. Draw the lewis structure for the formaldehyde (ch2o) molecule. Web how to draw the lewis dot structure for ch2o:
CH2O Lewis Structure Lewis Dot Structure for CH2O Methanal or
Count the valence electrons fro all atoms, and add or subtract electrons according to the charge. Looking at the periodic table, carbon has 4. In this structure, the carbon atom is the central atom, bonded to two hydrogen atoms and one oxygen atom. Hybridization of ch 2 o; Draw the lewis structure and answer the following question.
Select An Answer And Submit.
4 (c) + 2 (h) + 6 (o) = 12. Count the valence electrons fro all atoms, and add or subtract electrons according to the charge. Web how to draw lewis structure for ch 2 o; Draw the lewis structure for the formaldehyde (ch2o) molecule.
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each Atom.
Starting from this structure, complete the lewis structure that follows the octet rule on all atoms. The bonds are represented by drawing lines, whereas the electrons are represented by dots. (a) cs (b) bf4 (c) hno2 (where. Web draw the lewis structure for the formaldehyde (ch2o) molecule.
Determine The Total Number Of Electrons In The Carbon, Hydrogen.
I also go over hybridization, shape, sigma, pi bonding and bon. Be sure to include all resonance structures that satisfy the octet rule. Web arrange the steps involved in drawing a lewis structure in the correct order, starting with the first step on top. Web the compound formaldehyde has the molecular formula ch 2 o.
Be Sure To Include All Resonance Structures That Satisfy The Octet Rule.
There is a double bond between oxygen and carbon atom. The number of valence electrons for carbon is 4, oxygen is 6, and hydrogen is 1. Web formaldehyde lewis structure. Web how to draw the lewis dot structure for ch2o: