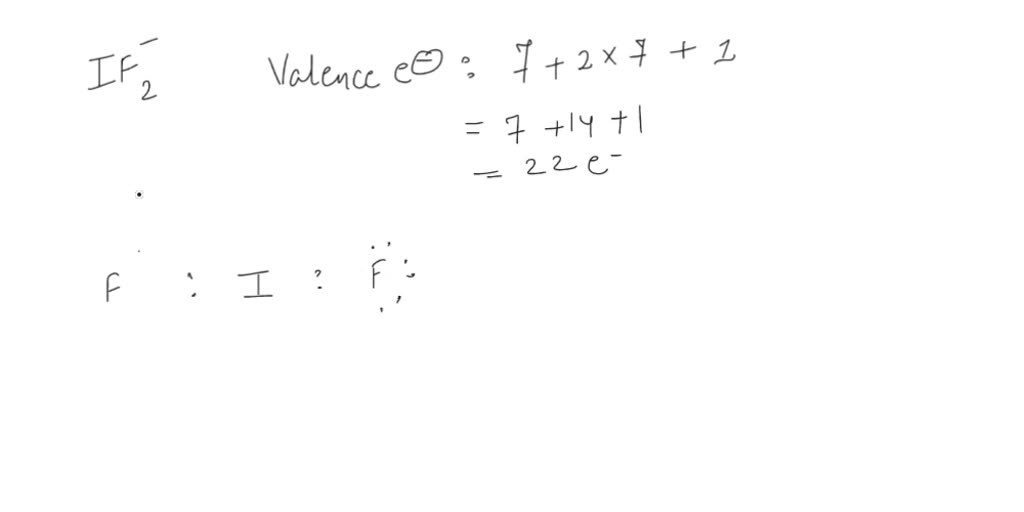Draw The Lewis Structure For The Iodine Difluoride Ion
Draw The Lewis Structure For The Iodine Difluoride Ion - Since it's an ion, we. Iodide is the name of the person. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Calculate the total number of valence electrons. Follow these simple steps to draw lewis dot structures: Hydrogen has an elected configuration. Draw the lewis structure for the iodine difluoride (if,) ion. Here’s the best way to solve it. Iodine (i) has 7 valence electrons, and each fluorine (f) atom has 7 valence electrons. Calculate the total number of valence electrons present in the iodine.
Where, v= no of valance electrons, b= no of. Follow these simple steps to draw lewis dot structures: Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses. Web may 23, 2023 by jay rana. Iodide is the name of the person. The structure of hydrogen has to be drawn. Web iodine gets the electron. The central atom in lewis structures is usually the least electronegative. Since it's an ion, we. 19k views 3 years ago.
Here’s the best way to solve it. Web draw lewis structures for ionic compounds. Iodine (i) has 7 valence electrons, and each fluorine (f) atom has 7 valence electrons. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining. Draw the lewis structure for the iodine difluoride (if,) ion. Draw the atoms on paper and put dots. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or. Iodide is the name of the person. 19k views 3 years ago. Since it's an ion, we.
SOLVED Draw the Lewis structure for the iodine difluoride (IF2 ion
Web drawing lewis dot structures and resonance structures. Calculate the total number of valence electrons present in the iodine. Web may 23, 2023 by jay rana. Count the total number of valence electrons: Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a.
Lewis Structure For Iodine
Count the total number of valence electrons: 19k views 3 years ago. Where, v= no of valance electrons, b= no of. Web may 23, 2023 by jay rana. Web drawing lewis dot structures and resonance structures.
Lewis Dot Diagram Iodine
The structure of hydrogen has to be drawn. Here’s how to approach this question. Calculate the total number of valence electrons. Follow these simple steps to draw lewis dot structures: The central atom in lewis structures is usually the least electronegative.
Lewis Structure For Iodine
The central atom in lewis structures is usually the least electronegative. Where, v= no of valance electrons, b= no of. 19k views 3 years ago. Draw lewis structures for covalent compounds. Draw the atoms on paper and put dots.
IF2 Lewis Structure How to Draw the Lewis Structure for IF2 YouTube
Iodine (i) has 7 valence electrons, and each fluorine (f) atom has 7 valence electrons. Web drawing lewis dot structures and resonance structures. Calculate the total number of valence electrons present in the iodine. The central atom in lewis structures is usually the least electronegative. Where, v= no of valance electrons, b= no of.
Solved Draw the Lewis structure for the iodine difluoride
Calculate the total number of valence electrons present in the iodine. The following procedure can be used to construct lewis electron structures for more complex. Here’s the best way to solve it. Draw the lewis structure for the iodine difluoride (if,) ion. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure).
(Get Answer) Draw The Lewis Structure For The Iodine Difluoride (IF 7
Since it's an ion, we. Follow these simple steps to draw lewis dot structures: In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining. Calculate the total number of valence electrons. Draw the lewis structure for the iodine difluoride (if,) ion.
SOLVED Draw the Lewis structure for the iodine difluoride ([Fz) ion.
Calculate the total number of valence electrons present in the iodine. Hydrogen has an elected configuration. Web drawing lewis dot structures and resonance structures. The central atom in lewis structures is usually the least electronegative. Draw the lewis structure for the iodine difluoride (if,) ion.
Iodine Electron Configuration (I) with Orbital Diagram
Follow these simple steps to draw lewis dot structures: A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Draw the lewis structure for the iodine difluoride (if,) ion. Since it's an ion, we. Count the total number of valence electrons:
In Section 4.7, We Demonstrated That Ions Are Formed By Losing Electrons To Make Cations, Or By Gaining.
Follow these simple steps to draw lewis dot structures: Here’s the best way to solve it. Here, the electrons of iodine would be distributed in the following way to complete the octet of the connected groups: The central atom in lewis structures is usually the least electronegative.
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Calculate the total number of valence electrons. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Where, v= no of valance electrons, b= no of.
Web Draw Lewis Structures For Ionic Compounds.
The following procedure can be used to construct lewis electron structures for more complex. Here’s how to approach this question. Calculate the total number of valence electrons present in the iodine. The structure of hydrogen has to be drawn.
19K Views 3 Years Ago.
The electronic configuration of kr four d. Count the total number of valence electrons: The negative charge on this ion indicates that it accepts an additional electron to form this structure. This widget gets the lewis structure of chemical compounds.