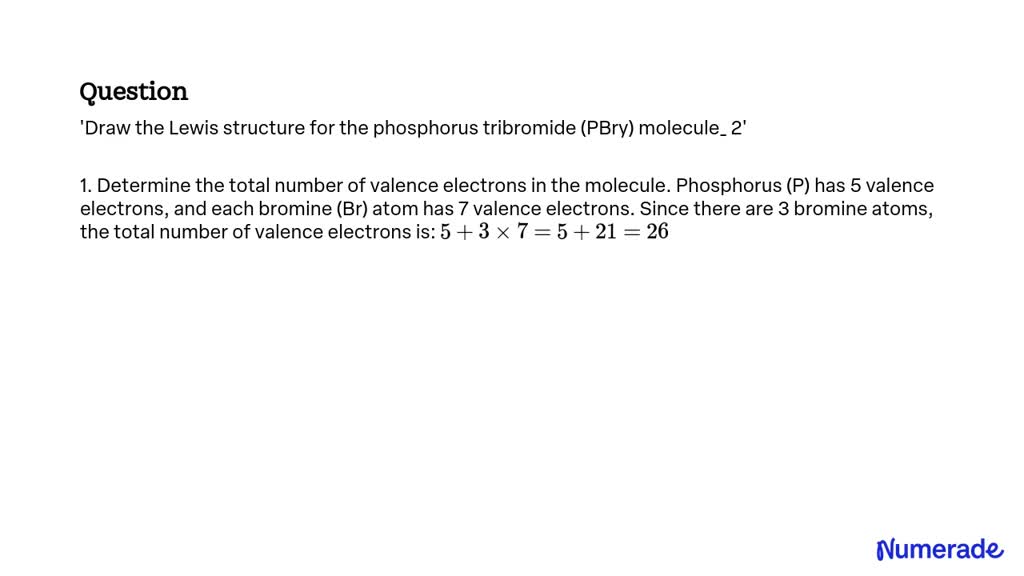
Draw The Lewis Structure For The Phosphorus Tribromide Molecule
Draw The Lewis Structure For The Phosphorus Tribromide Molecule - Web drawing lewis structures for molecules with one central atom: If you can do those lewis structures pbr 3 will be easy. In the lewis structure of pbr3, there are three bonding pairs of electrons and one lone pair of electrons on the central atom. Web hello guys!we are back with yet another video to help you determine the lewis structure of pbr3 or phosphorus bromide. Web to draw the lewis structure of (phosphorus tribromide), we need to determine the total number of valence electrons for the molecule. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Put two electrons between atoms to form a chemical bond. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web phosphorus tribromide (pbr3) consists of a central phosphorus (p) atom with 5 valence electrons, bonded to three bromine (br) atoms, each with 7 valence electrons. Steps for writing lewis structures.
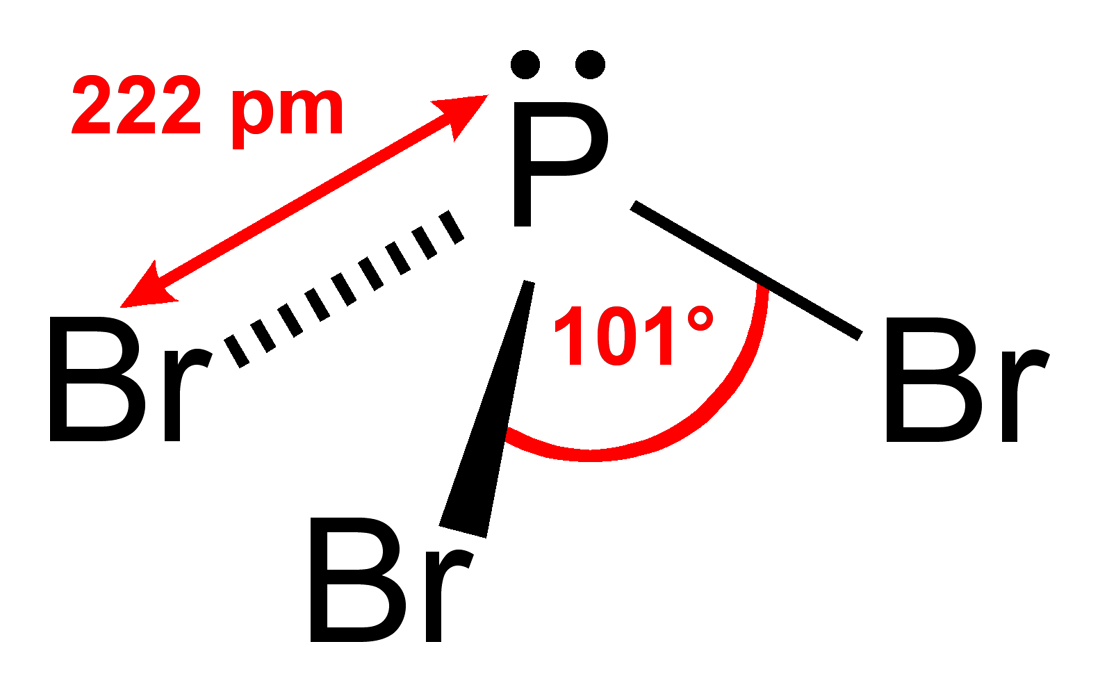
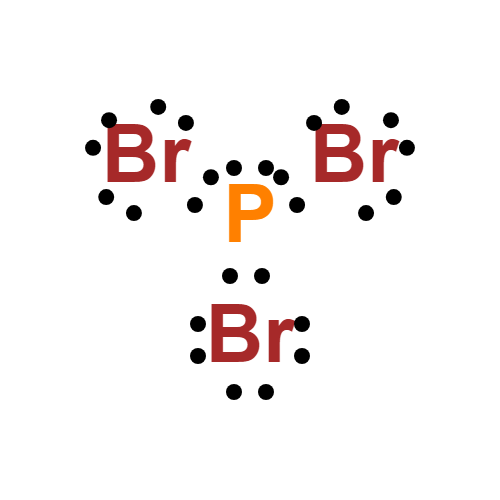
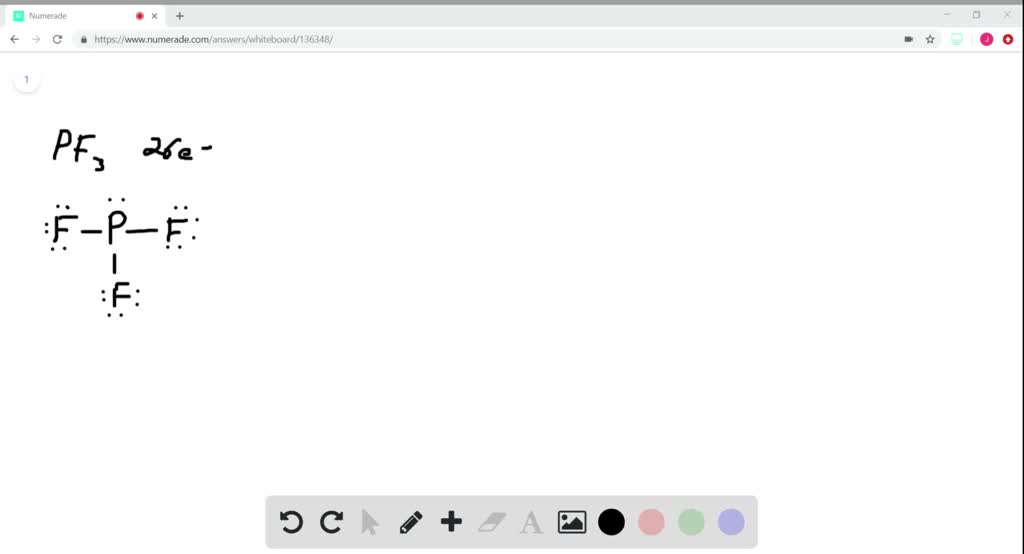
Determine the total number of electrons in the valence (outer shell). Web phosphorus tribromide (pbr3) consists of a central phosphorus (p) atom with 5 valence electrons, bonded to three bromine (br) atoms, each with 7 valence electrons. In the lewis structure for pbr 3 there are a total of 26 valence electrons. Web hello guys!we are back with yet another video to help you determine the lewis structure of pbr3 or phosphorus bromide. There is 1 lone pair on the phosphorus atom (p) and 3 lone pairs on all three bromine atoms (br). Nocl, cf 2 cl 2, hcn. Web to draw the lewis structure of (phosphorus tribromide), we need to determine the total number of valence electrons for the molecule. In order to draw the lewis structure of pbr3, first of all you have to find the total number of valence electrons present in the pbr3 molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web pbr3 lewis structure has a phosphorus atom (p) at the center which is surrounded by three bromine atoms (br).
#2 mention lone pairs on the atoms. (valence electrons are the number of electrons present in the outermost shell. Due to the presence of a vacant orbital of phosphorus atoms, it acts as lewis acid. #3 if needed, mention formal charges on the atoms. Draw the lewis structure for the phosphorus tribromide (pbry) molecule. This structure is also available as a 2d mol file. Here’s how you can easily draw the pbr 3 lewis structure step by step: In the periodic table, phosphorus is in the 15th group, and bromine is in the 17th. There are a total of 26 valence electrons for pbr3. Steps for writing lewis structures.
SOLVED 'Draw the Lewis structure for the phosphorus tribromide (PBry
1 (p) + 3 (br) = 1 (5) + 3 (7) = 26. Draw the lewis structure for the phosphorus tribromide (pbr3) molecule. Now, let’s take a closer look at each step mentioned above. (valence electrons are the number of electrons present in the outermost shell. Web pbr3 lewis structure has a phosphorus atom (p) at the center which is.
PBr3 Lewis Structure, Molecular Geometry, Hybridization and Polarity
This problem has been solved! Treichel, john townsend, david treichel. Draw the lewis structure for the phosphorus tribromide (pbry) molecule. #2 mention lone pairs on the atoms. Draw the lewis structure for the phosphorus tribromide (pbry) molecule.
Phosphorus tribromide 7789608
Web pbr3 lewis structure has a phosphorus atom (p) at the center which is surrounded by three bromine atoms (br). Web to draw the lewis structure of (phosphorus tribromide), we need to determine the total number of valence electrons for the molecule. Figure out how many electrons the molecule must have, based on the number of valence electrons in each..
Phosphorus tribromide wikidoc
There are 3 single bonds between the phosphorus atom (p) and each bromine atom (br). Web phosphorus tribromide (pbr3) consists of a central phosphorus (p) atom with 5 valence electrons, bonded to three bromine (br) atoms, each with 7 valence electrons. This widget gets the lewis structure of chemical compounds. In the lewis structure for pbr 3 there are a.
PBr3 Lewis Structure (Phosphorus Tribromide) in 2021 Lewis, Molecules
Web hello guys!we are back with yet another video to help you determine the lewis structure of pbr3 or phosphorus bromide. Web to draw the lewis structure of (phosphorus tribromide), we need to determine the total number of valence electrons for the molecule. This problem has been solved! Draw the lewis structure for the phosphorus. Find more chemistry widgets in.
Phosphorus tribromide, 99, Thermo Scientific Chemicals Fisher Scientific
In order to draw the lewis structure of pbr3, first of all you have to find the total number of valence electrons present in the pbr3 molecule. #1 draw a rough skeleton structure. Put the least electronegative atom in the center. Calculate the total number of valence electrons. This structure is also available as a 2d mol file.
SOLVED(a) Draw the dominant Lewis structure for the phosphorus
Ć с it х $ ? For the pbr3 structure use the periodic table to find the total number of valence. #3 if needed, mention formal charges on the atoms. Phosphorus has an atomic number of 15 and therefore has a valency of 5. Web hello guys!we are back with yet another video to help you determine the lewis structure.
SOLVED Draw the Lewis structure for the phosphorus tribromide (PBr
Due to the presence of a vacant orbital of phosphorus atoms, it acts as lewis acid. #2 mention lone pairs on the atoms. Determine the total number of electrons in the valence (outer shell). In the lewis structure of pbr3, there are three bonding pairs of electrons and one lone pair of electrons on the central atom. For the pbr3.
Draw the Lewis Structure for the Phosphorus Tribromide Pbr3 Molecule
Ć ć :0 | с 119 х 5 ? Web hello guys!we are back with yet another video to help you determine the lewis structure of pbr3 or phosphorus bromide. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web steps to draw lewis structure ofpbr 3, 1. #1 draw a rough skeleton.
SOLVED Draw the Lewis structure for the phosphorus tribromide (PBr
Phosphorus (p) is in group 5a and has 5 valence electrons, while each bromine atom (br) is in group 7a and has 7 valence electrons. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. There are a total of 26 valence electrons for pbr3. This structure is also available as a 2d mol file..
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Here, the given molecule is pbr3 (phosphorus tribromide). Find the total valence electrons for the molecule. In order to draw the lewis structure of pbr3, first of all you have to find the total number of valence electrons present in the pbr3 molecule. This structure is also available as a 2d mol file.
Laboratory Chemical Safety Summary (Lcss) Datasheet.
This problem has been solved! This problem has been solved! This widget gets the lewis structure of chemical compounds. Web hello guys!we are back with yet another video to help you determine the lewis structure of pbr3 or phosphorus bromide.
There Are A Total Of 26 Valence Electrons For Pbr3.
This structure is also available as a 2d mol file. In the lewis structure of pbr3, there are three bonding pairs of electrons and one lone pair of electrons on the central atom. There is 1 lone pair on the phosphorus atom (p) and 3 lone pairs on all three bromine atoms (br). Web draw the lewis structure for the phosphorus tribromide (pbr,) molecule.
Ć Ć :0 | С 119 Х 5 ?
Draw the lewis structure for the phosphorus tribromide (pbry) molecule. Web phosphorus tribromide comprises one phosphorus atom and three bromine atoms. Calculate the total number of valence electrons. It has sp3 hybridization, and the bond angle is approximately 109.5°.