Draw The Lewis Structure For The Sulfur Hexafluoride Molecule
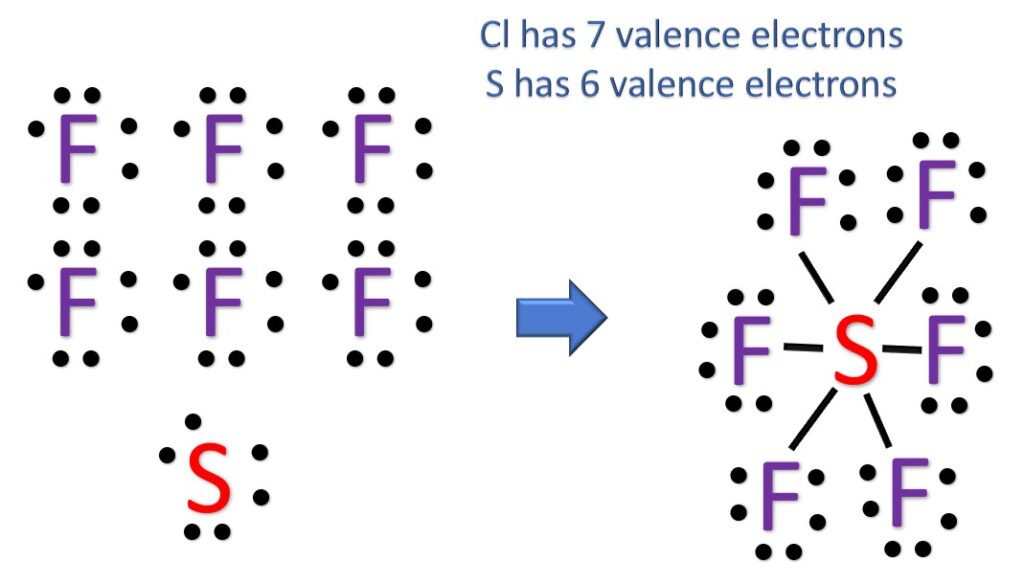
Draw The Lewis Structure For The Sulfur Hexafluoride Molecule - Calculate the total number of valence electrons. 69k views 12 years ago every video. The sulfur atom has six valence electrons, while each fluorine atom contributes one valence electron. Here’s how to approach this question. In order to draw the lewis structure of sf6, first of all you have to find the total number of valence electrons present in the sf6 molecule. 8 + (6 × × 7) = 50; Web sf6 + 4h2o → h2so4 + 6hf. Draw the lewis structure for the sulfur hexafluoride (sf_6) molecule. Indicate the hybridization (sp, sp’, sp, spºd, sp?d?) for each of the atoms indicated. Thus sf6 has 48 valence electrons that will help us draw the lewis dot structure of sf6.
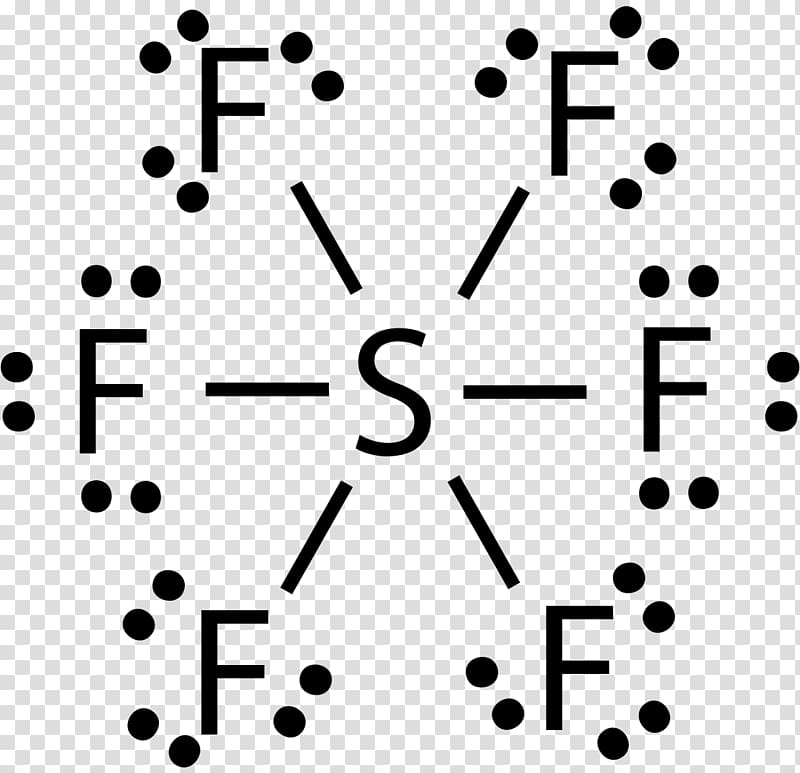
Web for the sf6 lewis structure there are a total of 12 valence electrons on the sulfur (s) atom. In this case, we can condense the last few steps, since not all of them apply. Try to draw the sf 6 lewis structure before watching the video. Identify the total number of valence electrons in the sulfur hexafluoride (sf6) molecule. There are 2 steps to solve this one. Here’s how to approach this question. In order to draw the lewis structure of sf6, first of all you have to find the total number of valence electrons present in the sf6 molecule. We’ll look at each stage of drawing the lewis structure of sulfur hexafluoride (sf6). 1.4k views 3 years ago lewis structure (chemistry) how to draw a lewis structure for sf6 (sulfur hexafluoride) lewis structure: Web to draw a lewis structure of a molecule, there are a few general steps to follow.
1.4k views 3 years ago lewis structure (chemistry) how to draw a lewis structure for sf6 (sulfur hexafluoride) lewis structure: Used in magnesium manufacturing as a cover gas and in certain semiconductor. 8 + (2 × × 7) = 22 xef 6: Here’s how to approach this question. 4k views 11 years ago chemistry lewis dot structures. We’ll look at each stage of drawing the lewis structure of sulfur hexafluoride (sf6). Sf6 + 8naoh → na2so4 + 6naf + 4h2o. 161k views 10 years ago. In order to find the total valence electrons in sf6 (sulfur hexafluoride) molecule, first of all you should know the valence electrons present in sulfur atom as well as fluorine atom. B) what is the molecular geometry?
Draw the Lewis structure for the sulfur hexafluoride SF6 molecule YouTube
Web in sf 6 lewis structure, each fluorine atom has made single bonds with center sulfur atom. This molecule comprises one sulf. Web to draw a lewis structure of a molecule, there are a few general steps to follow. 8 + (6 × × 7) = 50; A video explanation of how to draw the lewis dot structure for sulfur.
Sulfur Hexafluoride Lewis Structure
Try to draw the sf 6 lewis structure before watching the video. Find the total valence electrons in sf6 molecule. 100% (10 ratings) share share. 8 + (6 × × 7) = 50; Draw a skeleton joining the atoms by single bonds.
Sulfur Hexafluoride The Nightmare Greenhouse Gas That’s Just Too
Sf6 + 8naoh → na2so4 + 6naf + 4h2o. Web = 6 + 42. (6 points) ch3 en h3c .0: Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Web april 24, 2022 by aditi roy.
Sf6 sulfur hexafluoride molecule Royalty Free Vector Image
I quickly take you through how to draw the lewis structure of sf6 (sulfur hexafluoride). Web steps of drawing sf6 lewis structure step 1: There are three lone pairs on each fluorine atom, and the sulfur atom does not have any lone pair. 4k views 11 years ago chemistry lewis dot structures. Web hi guys !hi guys, today we are.
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity
Thus sf6 has 48 valence electrons that will help us draw the lewis dot structure of sf6. A video explanation of how to draw the lewis dot structure for sulfur. 100% (10 ratings) share share. Try to draw the sf 6 lewis structure before watching the video. In this tutorial, we will learn how to draw the lewis structure of.
Sulfur Hexafluoride Lewis Structure
Web the lewis structure of sf6 contains six single bonds, with sulfur in the center, and six fluorines on either side. It is helpful if you: Valence electrons of sulfur = 6, and valence electrons of fluorine = 7 there are 6 atoms of fluorine in this compound, so the total valence electrons of fluorine here are = 7*6 =.
Sulfur hexafluoride Electrical Test Tech
In order to find the total valence electrons in sf6 (sulfur hexafluoride) molecule, first of all you should know the valence electrons present in sulfur atom as well as fluorine atom. Web = 6 + 42. I quickly take you through how to draw the lewis structure of sf6 (sulfur hexafluoride). Thus sf6 has 48 valence electrons that will help.
SF6 Molecular Geometry,Shape and Bond Angles (Sulphur Hexafluoride
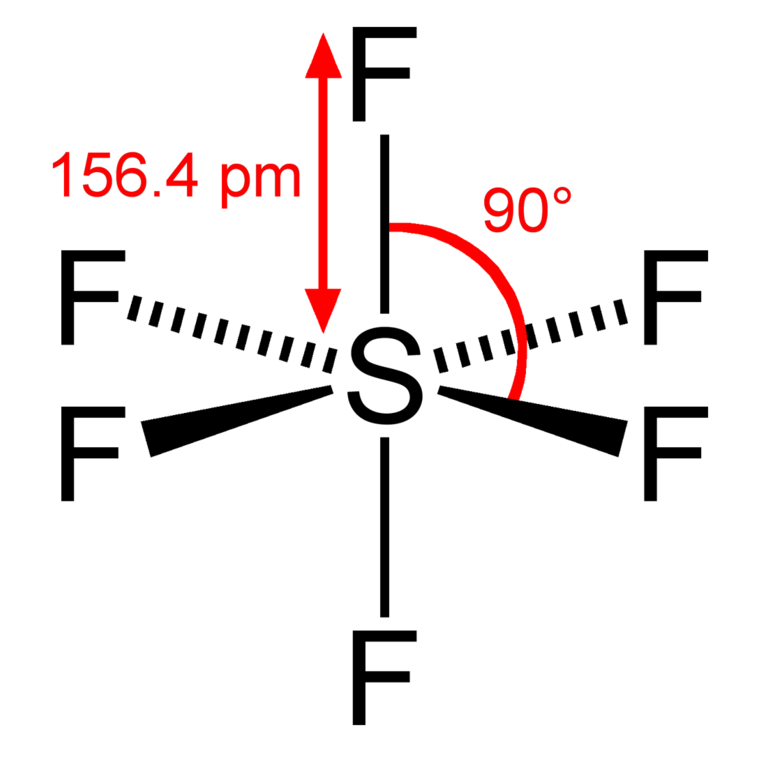
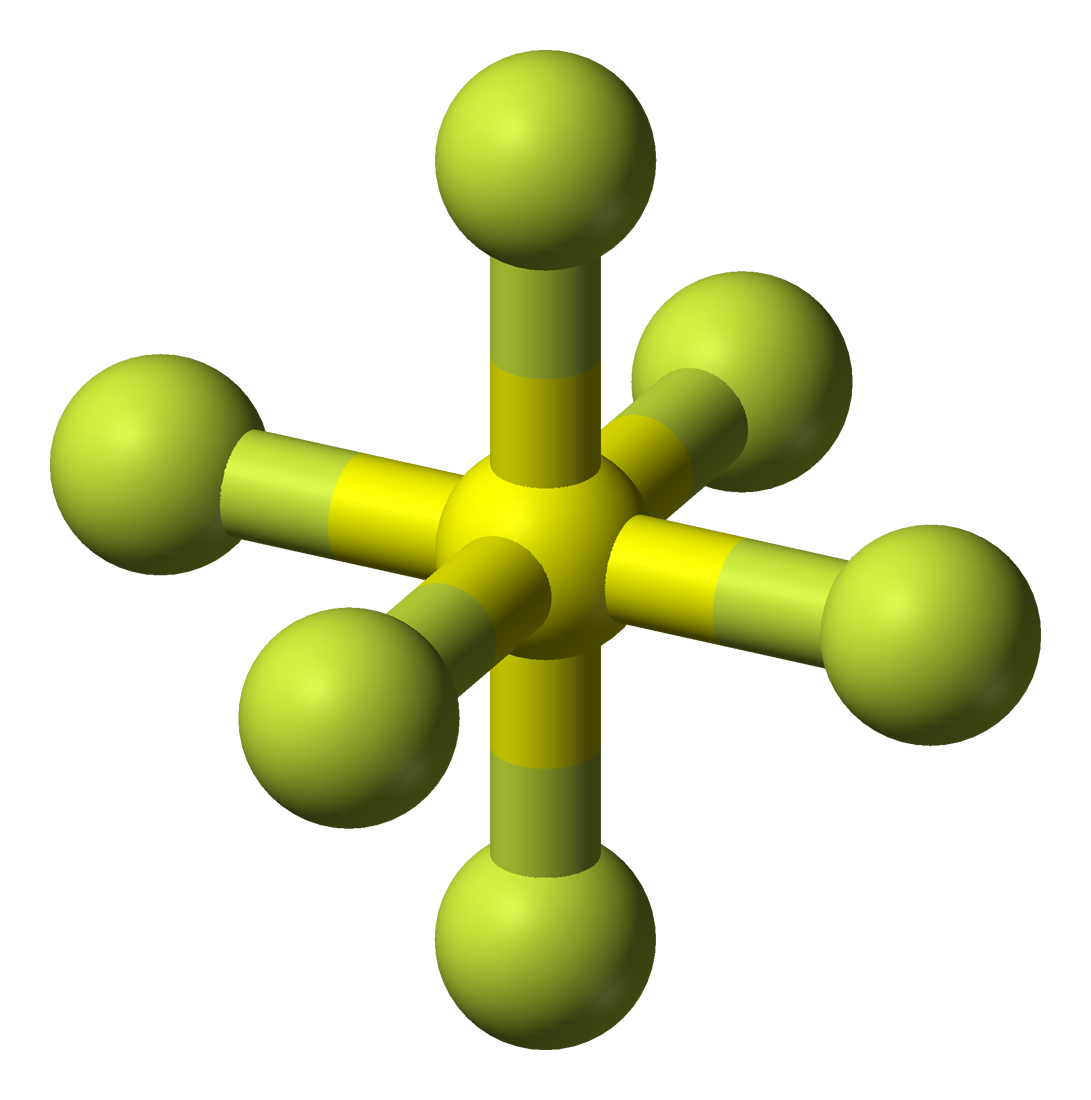
Sulphur hexafluoride reacts with a base like sodium hydroxide and forms sodium sulphate, sodium fluoride and water. 100% (10 ratings) share share. Draw the lewis structure of the sulfur hexafluoride molecular sf, and use the drawing to determine the molecule's geometry. In order to draw the lewis structure of sf6, first of all you have to find the total number.
SF6 (Sulfur hexafluoride) Lewis Structure
The lewis structure of sf6 ( sulfur hexafluoride) consists of a central sulfur atom bonded to six fluorine atoms. • how to draw lewis. Draw a skeleton joining the atoms by single bonds. Web sf6 + 4h2o → h2so4 + 6hf. Web hi guys !hi guys, today we are going to learn the lewis structure of sulfur hexafluoride having the.
Solved Draw the Lewis structure for the sulfur hexafluoride
Here, the given molecule is sf6 (sulfur hexafluoride). Find the total valence electrons in sf6 molecule. Web the lewis structure of sf6 contains six single bonds, with sulfur in the center, and six fluorines on either side. Web to draw a lewis structure of a molecule, there are a few general steps to follow. I also go over the hybridization,.
In Order To Draw The Lewis Structure Of Sf6, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Sf6 Molecule.
(valence electrons are the number of electrons present in the outermost shell of an atom). 1.4k views 3 years ago lewis structure (chemistry) how to draw a lewis structure for sf6 (sulfur hexafluoride) lewis structure: A video explanation of how to draw the lewis dot structure for sulfur. B) what is the molecular geometry?
In This Article, “Sf6 Lewis Structure”, Hybridization, Geometry, Formal Charge Along With Some Detailed Explanations Are Discussed Briefly.
Find the total valence electrons in sf6 molecule. Calculate the number of valence electrons: Here’s the best way to solve it. The structure of caffeine is shown.
Web Sf6 + 4H2O → H2So4 + 6Hf.
There are no lone pairs on sulfur atom and three lone pairs on each fluorine atom. (6 points) ch3 en h3c .0: Here, the given molecule is sf6 (sulfur hexafluoride). Sulphur hexafluoride reacts with a base like sodium hydroxide and forms sodium sulphate, sodium fluoride and water.
How To Draw The Lewis Structure For Sf6.
100% (10 ratings) share share. There are three lone pairs on each fluorine atom, and the sulfur atom does not have any lone pair. There are 2 steps to solve this one. 4k views 11 years ago chemistry lewis dot structures.