Draw The Lewis Structure For The Xenon Difluoride Molecule
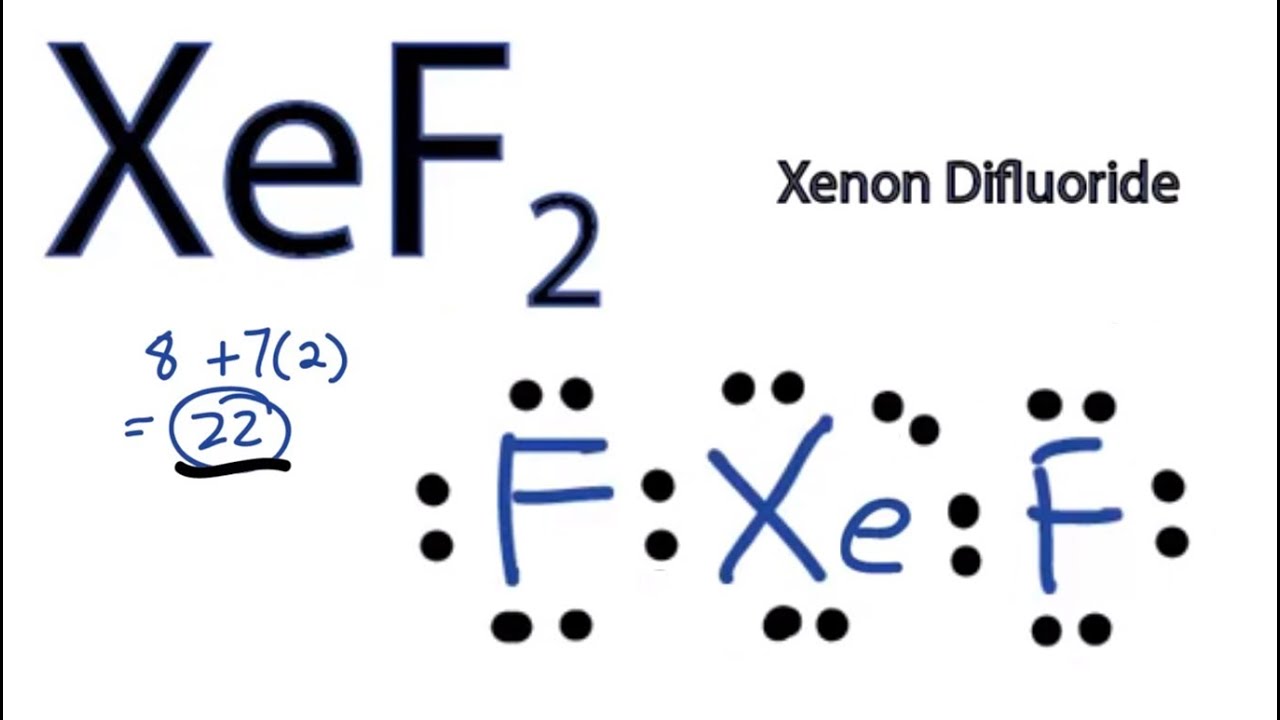
Draw The Lewis Structure For The Xenon Difluoride Molecule - Out of these compounds, xef2 is the most stable one. Xef2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. (valence electrons are the number of electrons present in the outermost shell of an atom). Web how to draw xef2 lewis structure. Chemistry questions and answers (a) (2 points) in the space provided below, draw a lewis structure for the molecule xenon difluoride, xef2. Web here’s the best way to solve it. Fluorine (atomic number = 9 and electronic configuration = 2,7) has 7 valence electrons. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for xenon difluoride (xef2). It is a powerful fluorinating as well as an oxidizing agent. Determine the total number of valence electrons.
Steps for drawing the xef2 lewis structure. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). It was discovered in the year 1962. Web in this article, we will discuss about xeo2f2 lewis structure, hybridization, formal charge, and its geometry. Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Web i quickly take you through how to draw the lewis structure of xef2 (xenon difluoride). Out of these compounds, xef2 is the most stable one. Web drawing the lewis structure for xef 2. For the xef2 lewis structure we first count the valence electrons for the xef2 molecule using the periodic table. 70 more lewis dot structures.
Drawing the lewis structure for xef 2. Web xef2 lewis structure, molecular geometry, hybridization, and mo diagram. Web solutions to example 10.4.1. It is a powerful fluoridating agent. Total valence electrons in xef2 molecule = valence electrons given by 1 xenon atom + valence electrons given by 2 fluorine atoms = 8 + 7(2) = 22. This is an active solvent and is found to be soluble in different fluorides like hf and bromine pentafluoride. Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Chemistry questions and answers (a) (2 points) in the space provided below, draw a lewis structure for the molecule xenon difluoride, xef2. The electronegativity of f is 4.0. Web drawing the lewis structure for xef 2.
The Lewis Structure Of Xef2 Understanding The Bonding Of Xenon
Web to get a molecule’s 3d shape, it’s important to know about bond angles. Draw the lewis structure for the. Web here’s the best way to solve it. Web drawing the lewis structure for xef 2. In order to draw the lewis structure of xef2, first of all you have to find the total number of valence electrons present in.
XeO2F2 Lewis Structure How to Draw The Lewis Structure for XeO2F2
Web xef2 lewis structure, molecular geometry, hybridization, and mo diagram. Xe does not follow the octet rule. Fluorine (atomic number = 9 and electronic configuration = 2,7) has 7 valence electrons. See an example of a molecule that violates the octet rule (xef₂) and learn how to draw its lewis diagram in this video. Each h atom (group 1) has.
Xenon Difluoride Geometry
This is an active solvent and is found to be soluble in different fluorides like hf and bromine pentafluoride. Once we know how many valence electrons there are in xef2 we can distribute them around the central atom and attempt to fill the outer shells of. When constructing a lewis diagram, keep in mind the octet rule, which refers to.
xenon difluoride lewis structure.xef2.XeF2.lewis structure. YouTube
Xef 2 was considered to be a possible convenient replacement for elemental fluorine especially in addition reactions to a double bond. Fluorine (atomic number = 9 and electronic configuration = 2,7) has 7 valence electrons. Determine the total number of valence electrons. Step 1 calculate the number of valence electrons for xe and f. 70 more lewis dot structures.
Xenon Difluoride Geometry
Web xef2 lewis structure, molecular geometry, hybridization, and mo diagram. Xef 6 +2h 2 o —>. As explained above xenon difluoride is a symmetrical molecule with a linear shape and. Drawing the lewis structure for xef 2. Xenon having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8.
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 YouTube
Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Web a video explanation of how to draw the lewis dot structure for xenon difluoride, along with information about the compound including formal charges, polarity. Web solutions to example 10.4.1. Web chemistry learning made.
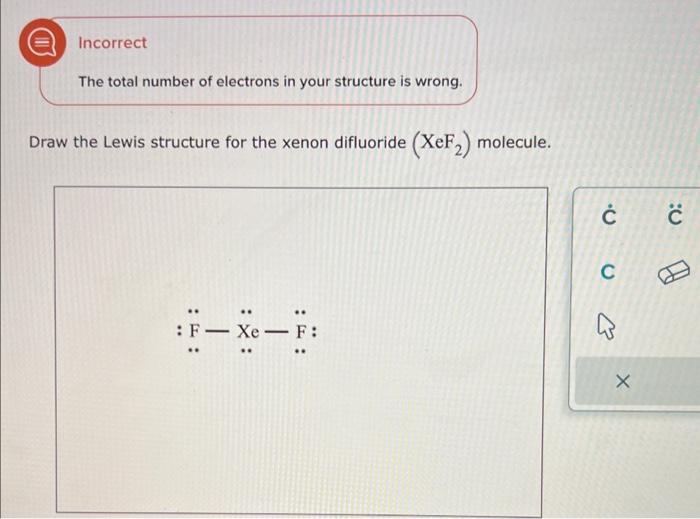
Solved Draw the Lewis structure for the xenon difluoride
Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Step 1 calculate the number of valence electrons for xe and f. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. 70 more lewis dot structures. Steps for drawing the xef2.
How to Draw the Lewis Dot Structure for Xenon (Xe) YouTube
Fluorine (atomic number = 9 and electronic configuration = 2,7) has 7 valence electrons. Determine the total number of valence electrons available for bonding in the xenon difluoride () molecule. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. It will hold more than 8 electrons. It is a powerful fluorinating as.
XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). Xe does not follow the octet rule. (b) (2 points) c the electronegativity of xe is 2.6. It was discovered in the year 1962. It is a powerful fluorinating as well as an oxidizing agent.
xenon difluoride Overview, Structure, Properties & Uses
Web drawing the lewis structure for xef 2. Out of these compounds, xef2 is the most stable one. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Total valence electrons in xef2 molecule = valence electrons given by 1 xenon atom + valence electrons given by 2 fluorine atoms =.
Once We Know How Many Valence Electrons There Are In Xef2 We Can Distribute Them Around The Central Atom And Attempt To Fill The Outer Shells Of.
This is an active solvent and is found to be soluble in different fluorides like hf and bromine pentafluoride. Xenon dioxide difluoride, sometimes known as xeo 2 f 2, is an inorganic molecule with the chemical formula xeo 2 f 2.the partial hydrolysis of xenon hexafluoride produces it, as shown in the following reaction: Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). Fluorine (f) is in group 17 and has 7 valence electrons each.
It Is A Powerful Fluorinating As Well As An Oxidizing Agent.
Web drawing the lewis structure for xef 2. Step 1 calculate the number of valence electrons for xe and f. It will hold more than 8 electrons. Xenon (xe) is in group 18 of the periodic table and has 8 valence electrons.
Steps For Writing Lewis Structures.
If we look at the process of synthesis of xenon. Determine the total number of valence electrons. Drawing the lewis structure for xef 2. Out of these compounds, xef2 is the most stable one.
Web How To Draw Xef2 Lewis Structure.
It was discovered in the year 1962. Total valence electrons in xef2 molecule = valence electrons given by 1 xenon atom + valence electrons given by 2 fluorine atoms = 8 + 7(2) = 22. Web to get a molecule’s 3d shape, it’s important to know about bond angles. (b) (2 points) c the electronegativity of xe is 2.6.