Draw The Lewis Structure Of Ch3Br
Draw The Lewis Structure Of Ch3Br - For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. • dipole moment of ch3br is 1.83d. Determine the central atom in the molecule. (valence electrons are the electrons that are. • the hybridization state of ch3br is sp 3. C p opy uste с chemdoodle. Explicitly draw all h atoms. Draw the molecule by placing atoms on the grid and connecting them with bonds. Predict the major products of this organic reaction: Here’s the best way to solve it.
Predict the major products of this organic reaction: Highlight the appropriate bond (s) by selecting the highlight. Web hello guys, welcome back to our channel, and in today's video, we share our quick and easy method to determine the lewis structure of the ch3br molecule. Put two between atoms to form chemical bonds. Web bromomethane, commonly known as methyl bromide, is an organobromine compound with formula c h 3 br.this colorless, odorless, nonflammable gas is produced both industrially and biologically. • include all valence lone pairs in your answer. There are several steps to draw the lewis structure of ch 3 br. Draw the molecule by placing atoms on the grid and connecting them with bonds. You can change your selection by clicking or tapping on any part of the drawing, including the elements and bonds. Count the total number of valence electrons for all the atoms in the molecule.
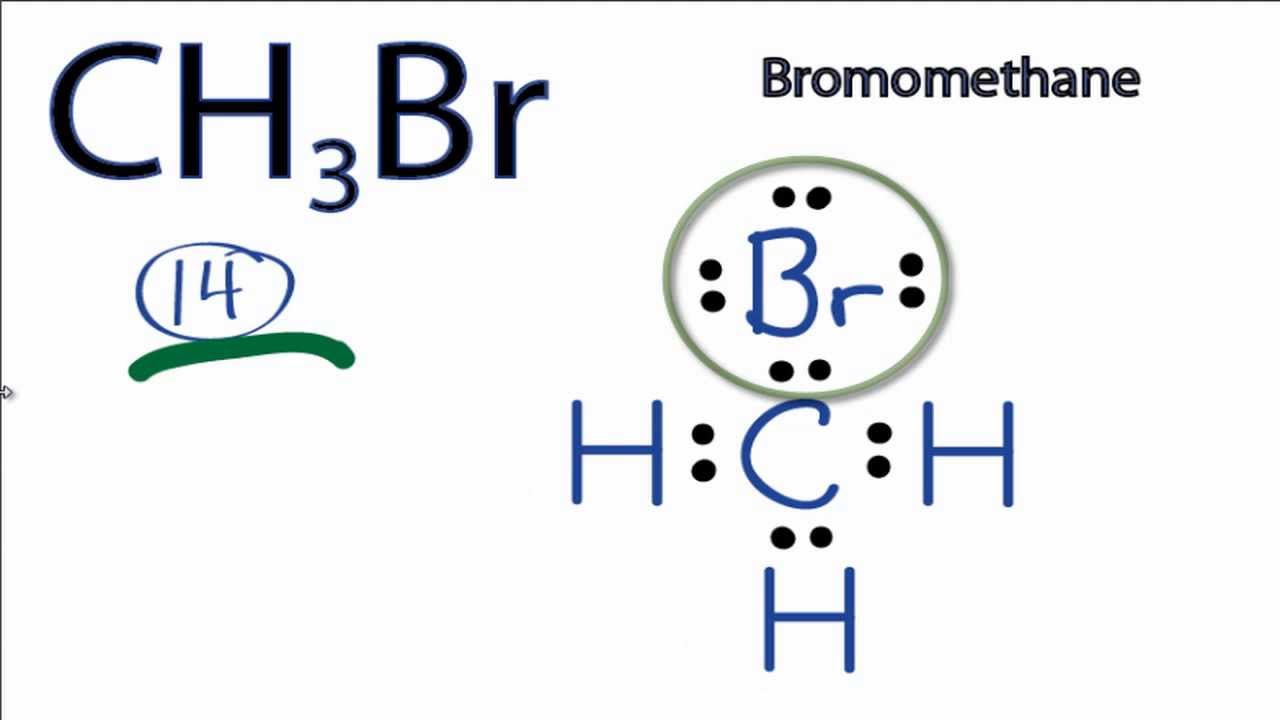
Web for ch3br, there are a total of 14 valence electrons. Hydrogen always goes on the outside, and since carbon is less electronegative than bromine, we'll p. Draw the lewis structure of ch₃br and then determine if the molecule is polar or nonpolar. You can change your selection by clicking or tapping on any part of the drawing, including the elements and bonds. Cations are formed when atoms lose electrons, represented by fewer lewis dots, whereas anions are formed by atoms gaining electrons. So the total number of valence electrons in ch3br is (4 + 3*1 + 7) = 14. Put two between atoms to form chemical bonds. Here’s the best way to solve it. • draw only the major product, or products. Web just click or tap on the plus symbol to open the drawing tool.
Draw The Lewis Structure Of Ch3Br at Drawing
Explicitly draw all h atoms. However, it is also known to occur in oceans in a small amount. 100% (2 ratings) share share. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. • draw only the major product, or products.
Chbr3 Lewis Structure
Determine total electrons pairs as lone pairs and bonds. • dipole moment of ch3br is 1.83d. For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. Calculate the total number of valence electrons. Web for ch3br, there are a total of 14 valence electrons.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
Find the total valence electrons in ch3br molecule. For ch3br, there are a total of 14 valence electrons. In the ch 3 br lewis structure, there are four single bonds around the carbon atom, with three hydrogen atoms and one bromine atom attached to it, and on the bromine atom, there are three lone pairs. C p opy uste с.
[Solved] Draw the Lewis structure of bromoform (CH3Br). a. What is the
Web check me out: (valence electrons are the electrons that are. Web just click or tap on the plus symbol to open the drawing tool. Here, the given molecule is ch3br. 100% (2 ratings) share share.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
Here’s the best way to solve it. Include lone pairs of electrons. Web steps of drawing ch3br lewis structure step 1: Determine total electrons pairs as lone pairs and bonds. We have 14 valence electrons for the ch3br lewis structure.
draw the lewis structure of ch3br howtohangcurtainsoverpatiodoor
There are several steps to draw the lewis structure of ch 3 br. It was used extensively as a pesticide until being phased out by most countries in the early 2000s. Web 6 steps to draw the lewis structure of ch3br step #1: (valence electrons are the electrons that are. Predict the major products of this organic reaction:
CH3Br Lewis Structure (Bromomethane) YouTube
Cl + + ☑ ho click and drag to start drawing a structure. Web the lewis structure of ch3br is: Web figure 13.4.2 13.4. Highlight the appropriate bond (s) by selecting the highlight. Draw the lewis structure of ch3br.
Draw The Lewis Structure Of Ch3br
• dipole moment of ch3br is 1.83d. Hydrogen always goes on the outside, and since carbon is less electronegative than bromine, we'll put the carbon in the center and the bromine on top. 100% (2 ratings) share share. 2 demonstrates the use of lewis symbols to show the transfer of electrons during the formation of ionic compounds. Predict the major.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
Web just click or tap on the plus symbol to open the drawing tool. Web draw the lewis structure of ch3br. Put two between atoms to form chemical bonds. Web 6 steps to draw the lewis structure of ch3br step #1: 2 demonstrates the use of lewis symbols to show the transfer of electrons during the formation of ionic compounds.
Chbr3 Lewis Structure
Include all lone pairs of electrons. Web 6 steps to draw the lewis structure of ch3br step #1: Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. C p opy uste с chemdoodle. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to.
It Was Used Extensively As A Pesticide Until Being Phased Out By Most Countries In The Early 2000S.
Count the total number of valence electrons for all the atoms in the molecule. Web steps of drawing ch3br lewis structure step 1: Explicitly draw all h atoms. Here, the given molecule is ch3br.
• Draw Only The Major Product, Or Products.
Part a for the ch3br molecule (c is the central atom), draw the correct lewis structure and select all polar covalent bonds. Find total number of electrons of the valance shells of hydrogen, oxygen atoms and carbon atom. Web draw the lewis structure ch3br, what is the hybridization for the catom? However, it is also known to occur in oceans in a small amount.
Find The Total Valence Electrons In Ch3Br Molecule.
Active or selected elements in the structure or bond appear brighter in red. The final answer must have this number of electrons‼! Here’s the best way to solve it. Highlight the appropriate bond (s) by selecting the highlight.
Web Bromomethane, Commonly Known As Methyl Bromide, Is An Organobromine Compound With Formula C H 3 Br.this Colorless, Odorless, Nonflammable Gas Is Produced Both Industrially And Biologically.
Include lone pairs of electrons. • include all valence lone pairs in your answer. Write a lewis structure for methyl bromide, ch3br, showing all valence electrons. Here’s the best way to solve it.