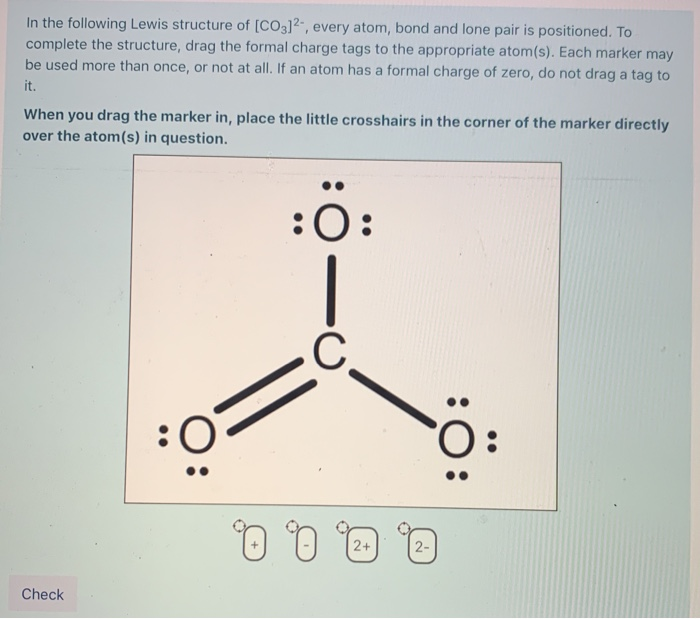
Draw The Lewis Structure Of Co32
Draw The Lewis Structure Of Co32 - Web sometimes one lewis structure is not enough. In this structure, the carbon atom is connected to three oxygen atoms, with one double bond and two single bonds. For example, drawing one lewis structure for ozone (o 3) gives us a misleading picture of the actual bonding in the molecule.if we draw a lewis structure for o 3 (ozone), we get this:. This video discusses the resonance structu. Starting from this structure, complete the lewis structure that follows the octet rule on all atoms. Carbon is the least electronegative, put that at. Draw a lewis structure for each of the following molecules or ions: Web the lewis structure for ammonia (nh₃) shown below is incorrect. (valence electrons are the number of electrons present in the. The remaining electrons are distributed as lone pairs on the oxygen atoms.
Draw the lewis structure of carbonate ion (co32) showing all possible resonance structures if there are any. The negative charge on the ion is located on two of the oxygen atoms, to indicate. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Obeys the octet rule b. In this case, carbon (c) contributes 4 valence electrons, and each oxygen (o) contributes 6 valence electrons, giving a total of 24 valence. This is done by dividing the total number of valence electrons by two. Lewis structure of carbonate ion is drawn in this tutorial step by step. This video discusses the resonance structu. Determine the formal charge of each atom. = 4 + 6*3 + 2.
This video discusses the resonance structu. Web sometimes one lewis structure is not enough. Determine the formal charge of each atom. In carbonate ion, among the two elements, carbon has an. Draw the lewis structure of ozone (o3) showing all possible resonance structures if there are any. The remaining electrons are distributed as lone pairs on the oxygen atoms. (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons (ve), and shared pairs (sp). Now, in order to draw the lewis structure, we have to determine which one is the central atom in a multiatomic heterogeneous molecule, here an ion. Web draw the lewis structure of the carbonate ion, co 32−. Web chemistry questions and answers.
Co3 2 Lewis Structure Braineds
You will learn about these facts in this tutorial. Web the lewis structure for ammonia (nh₃) shown below is incorrect. In this structure, the carbon atom is connected to three oxygen atoms, with one double bond and two single bonds. Determine the formal charge of each atom. Some molecules or ions cannot be adequately described by a single lewis structure.
Lewis Structure for CO3 2 (Carbonate ion) YouTube
Single bonds and 1c =o double. This video discusses the resonance structu. Some molecules or ions cannot be adequately described by a single lewis structure. In carbonate ion, among the two elements, carbon has an. Describe one resonance structure of the carbonate ion.
Draw the Lewis Structure for Co32
4 + (3 × 6) + 2 = 24 electrons. Web see the big list of lewis structures. Single bonds and 1c =o double. This video discusses the resonance structu. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2).
How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry
Since there are three oxygen atoms, the total number of valence electrons is: Obeys the octet rule b. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Determine the central atom of the molecule. Draw the lewis structure of carbonate ion (co32) showing all possible resonance structures if there are any.
CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2
The formal charge is a hypothetical charge from the dot structure. Oxygen has six, we have 3 oxygens, and this negative 2 means we have an extra two valence electrons. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2).
Draw the Lewis structure for CO3^2
Web see the big list of lewis structures. Determine the formal charge of each atom. (valence electrons are the number of electrons present in the. Web sometimes one lewis structure is not enough. This video discusses the resonance structu.
Co3 2 Lewis Structure Braineds
You will learn about these facts in this tutorial. This is done by dividing the total number of valence electrons by two. (a) cs (b) bf4 (c) hno2 (where. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Starting from this structure, complete the lewis structure that follows the octet rule on all atoms.
How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube
Six electrons are used, and 6 are left over. Obeys the octet rule b. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: (valence electrons are the number of electrons present in the. For example, drawing one lewis structure for ozone (o 3) gives us a misleading picture of the actual bonding in the.
CO32 Lewis Structure, Characteristics 13 Facts You Should Know
This video discusses the resonance structu. Starting from this structure, complete the lewis structure that follows the octet rule on all atoms. In this case, carbon (c) contributes 4 valence electrons, and each oxygen (o) contributes 6 valence electrons, giving a total of 24 valence. Oxygen has six, we have 3 oxygens, and this negative 2 means we have an.
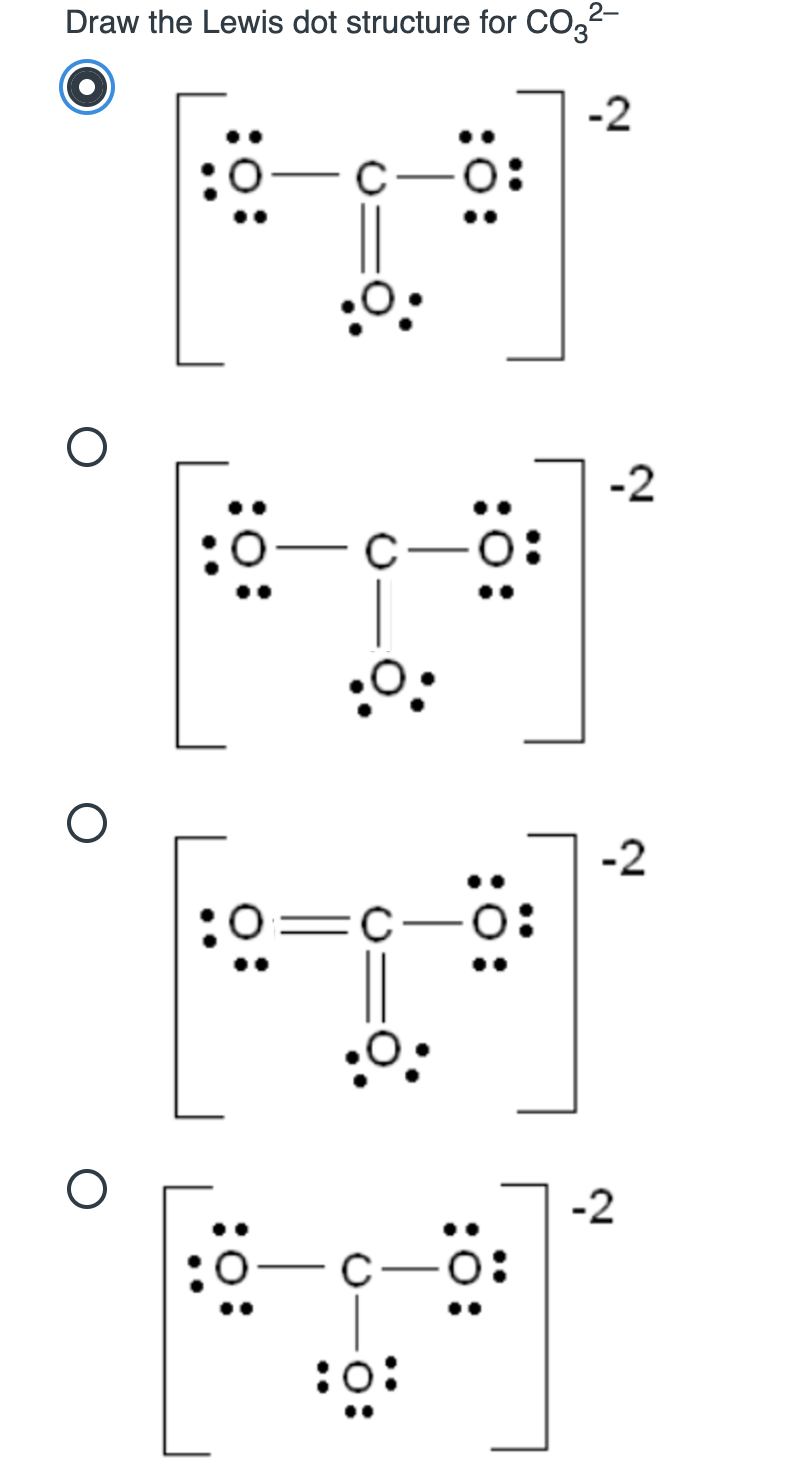
Solved Draw the Lewis dot structure for CO32 2 0 O 2 0
4 plus 18 plus 2: For example, drawing one lewis structure for ozone (o 3) gives us a misleading picture of the actual bonding in the molecule.if we draw a lewis structure for o 3 (ozone), we get this:. 4 + (3 × 6) + 2 = 24 electrons. Determine the total number of valence electrons. Some molecules or ions.
Draw The Lewis Structure Of Carbonate Ion (Co32) Showing All Possible Resonance Structures If There Are Any.
Start by counting the total number of valence electrons in the molecule/ion. Draw the lewis structure for co32, including any valid resonance structures. Determine the total number of valence electrons. The central carbon atom a.
In Carbonate Ion, Among The Two Elements, Carbon Has An.
This video discusses the resonance structu. You will learn about these facts in this tutorial. 4 + (3 × 6) + 2 = 24 electrons. = 4 + 6*3 + 2.
Now, In Order To Draw The Lewis Structure, We Have To Determine Which One Is The Central Atom In A Multiatomic Heterogeneous Molecule, Here An Ion.
Web sometimes one lewis structure is not enough. Describe one resonance structure of the carbonate ion. For example, drawing one lewis structure for ozone (o 3) gives us a misleading picture of the actual bonding in the molecule.if we draw a lewis structure for o 3 (ozone), we get this:. Web the lewis structure for ammonia (nh₃) shown below is incorrect.
Lewis Structure Of Carbonate Ion Is Drawn In This Tutorial Step By Step.
The remaining electrons are distributed as lone pairs on the oxygen atoms. Draw the lewis structure of ozone (o3) showing all possible resonance structures if there are any. Oxygen has six, we have 3 oxygens, and this negative 2 means we have an extra two valence electrons. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons.