Draw The Lewis Structure Of No2
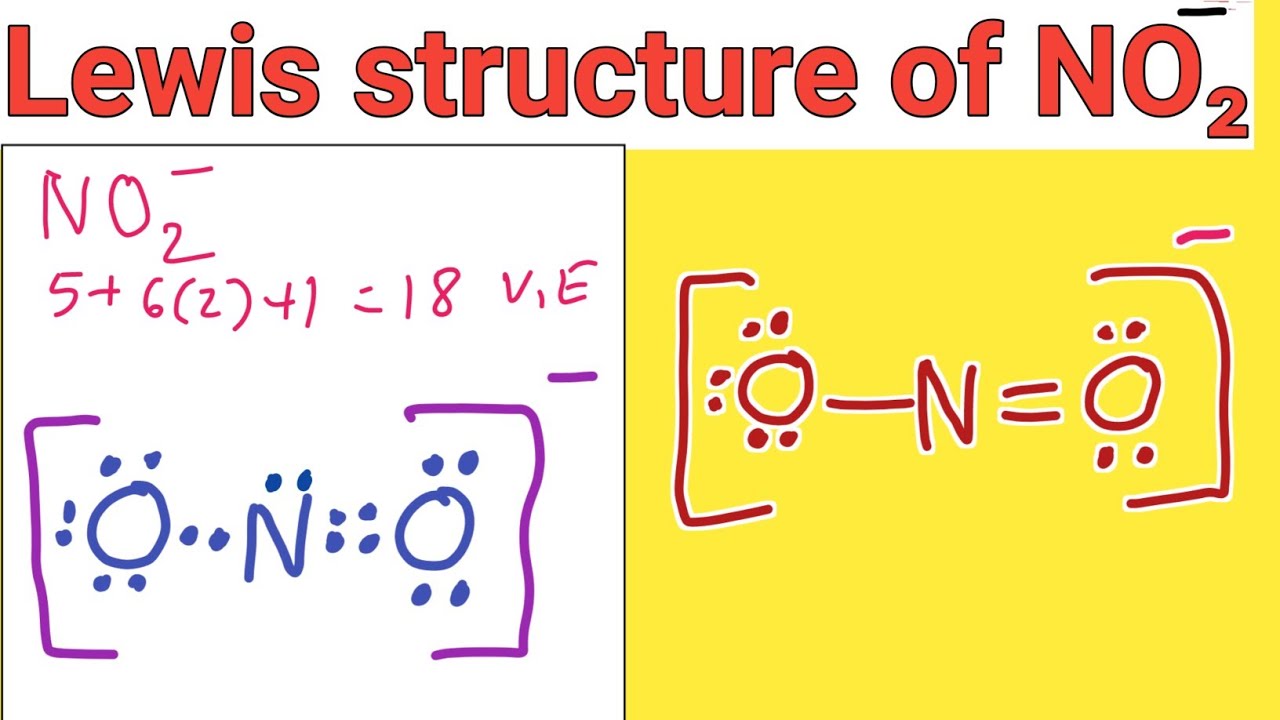
Draw The Lewis Structure Of No2 - Web let us talk about drawing the lewis structure for nitrogen dioxide ( no2 ). Describe the resonance hybrid of the nitrite ion. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. The lewis structure of no 2 molecule is shown below. Following steps are required to draw the no 2 lewis structure and they are explained in detail in this tutorial. For nitrogen we have 5 valence electrons; The neutral species that contain an unpaired electron is called radical (or free radical). The no2 lewis structure has a total of 17 valence. 216k views 6 years ago.
For the no2 structure use the. Watch the video and see if you missed any steps or information. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Web drawing the lewis structure for no 2 (nitrogen dioxide) viewing notes: For nitrogen we have 5 valence electrons; Put lone pairs on atoms Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Put lone pairs on atoms. (valence electrons are the number of electrons present in the outermost shell of an atom). Web steps of drawing no 2 lewis structure.
It describes the arrangement of valence electrons around particular atoms in a molecule. When the carbon atom of a. For the no+ structure use the periodic. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Watch the video and see if you missed any steps or information. This widget gets the lewis structure of chemical compounds. Draw the lewis structure of no2−. The no2 lewis structure has a total of 17 valence. Web figure 1.2g no molecule lewis structure. Web steps of drawing no 2 lewis structure.
Lewis structure of NO2 (Nitrite ion) Trick to draw Lewis dot
Nitrogen will only have 7 valence electrons but this. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (valence electrons are the electrons that are present in the outermost orbit of any atom.) Try to draw the no 2 lewis structure before watching the video. There are 3 steps to solve this one.
NO2 Lewis Structure How to Draw the Lewis Structure for NO2 YouTube
A lewis structure, also known as an electron dot structure, is a simplified representation of a molecule's valence shell electrons. Web to draw the lewis structure of no2 (nitrogen dioxide), start by placing the nitrogen atom in the center. Then, add a double bond between the nitrogen atom and one of the oxygen atoms. The following procedure will give you.
Lewis structure of NO2. How to draw the Lewis structure of NO2. Advance
Web how do you draw the lewis structure for no 2? Draw the lewis structure of no2−. For the no2 structure use the. Calculate the total number of valence electrons. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5.
How do you draw the lewis structure for NO2?
16k views 1 year ago. (valence electrons are the number of electrons present in the outermost shell of an atom). It describes the arrangement of valence electrons around particular atoms in a molecule. Web drawing lewis structures for molecules with one central atom: Describe the resonance hybrid of the nitrite ion.
NO2 Lewis Structure (Nitrite Ion) YouTube
Then, add a double bond between the nitrogen atom and one of the oxygen atoms. Send feedback | visit wolfram|alpha. 6 for oxygen, but we have two oxygens so we'll multiply that by two; (valence electrons are the number of electrons present in the outermost shell of an atom). Connect the nitrogen atom to two oxygen atoms using single bonds.
Lewis Structure of NO2(1), the nitrite ion. YouTube
It is helpful if you: Web figure 1.2g no molecule lewis structure. Here, the given molecule is no2 (nitrogen dioxide). Connect the nitrogen atom to two oxygen atoms using single bonds. 500k views 10 years ago.
NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
It is helpful if you: Put lone pairs on atoms. Let us look at the periodic table. Here, the given molecule is no2 (nitrogen dioxide). 500k views 10 years ago.
How to draw NO2+ Lewis Structure? Science Education and Tutorials
Web drawing the lewis structure for no 2 (nitrogen dioxide) viewing notes: Figure 1.2h no2 molecule lewis structure. 25k views 3 years ago lewis structures. Plus one for this valence electron up here; Find more chemistry widgets in wolfram|alpha.
How to draw the Lewis structure of NO2 + (Nitronium ion) YouTube
Web drawing the lewis structure for no 2 (nitrogen dioxide) viewing notes: Draw the lewis structure of no2−. For above molecules, they all contain unpaired (single) electrons. #lewis structure of no2 #no2 lewis structure # today i am going to explain how to draw the lewis structure for no2. It describes the arrangement of valence electrons around particular atoms in.
Then, Add A Double Bond Between The Nitrogen Atom And One Of The Oxygen Atoms.
Try structures similar to no 2 for more practice. Put lone pairs on atoms. For the no+ structure use the periodic. Try to draw the no 2 lewis structure before watching the video.
It Describes The Arrangement Of Valence Electrons Around Particular Atoms In A Molecule.
For above molecules, they all contain unpaired (single) electrons. In order to draw the lewis structure of no2, first of all you have to find the total number of valence electrons present in the no2 molecule. 16k views 1 year ago. Following steps are required to draw the no 2 lewis structure and they are explained in detail in this tutorial.
Center Atom Selection From Nitrogen And Oxygen Atom.
The no2 lewis structure has a total of 17 valence. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This widget gets the lewis structure of chemical compounds. This problem has been solved!
Connect The Nitrogen Atom To Two Oxygen Atoms Using Single Bonds.
Web steps of drawing no 2 lewis structure. (valence electrons are the electrons that are present in the outermost orbit of any atom.) How to draw lewis structures: Let us look at the periodic table.