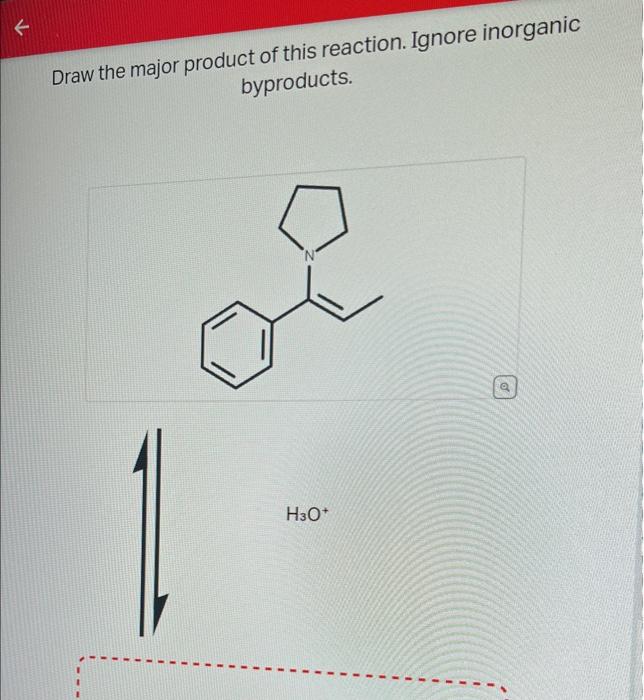
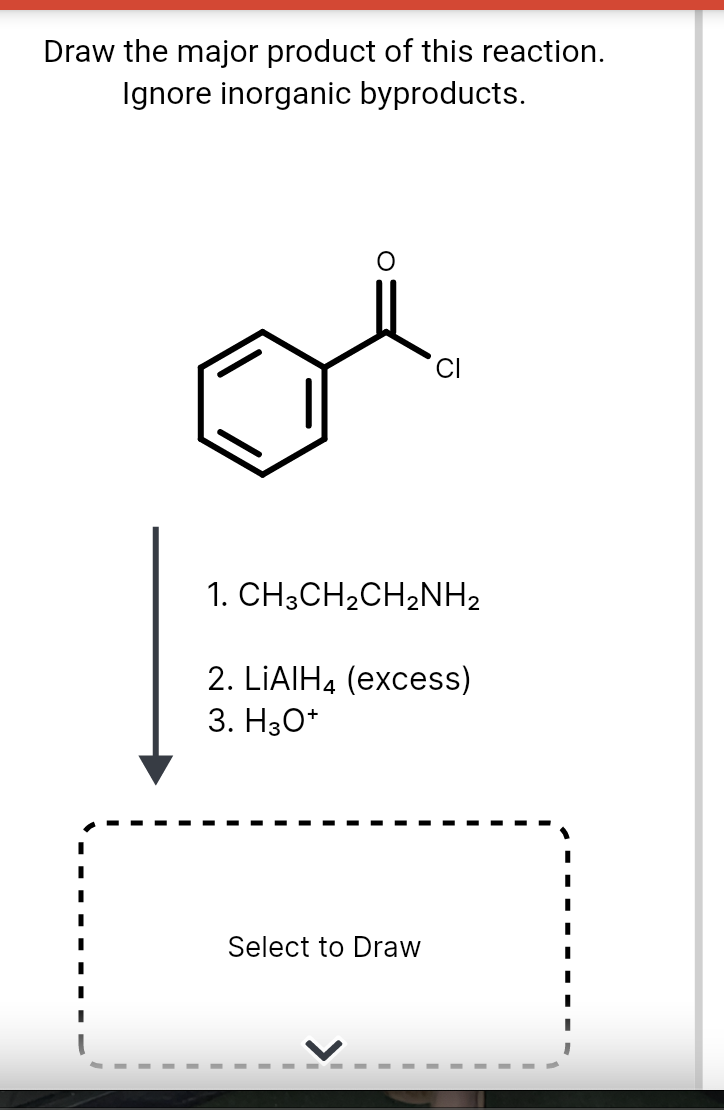
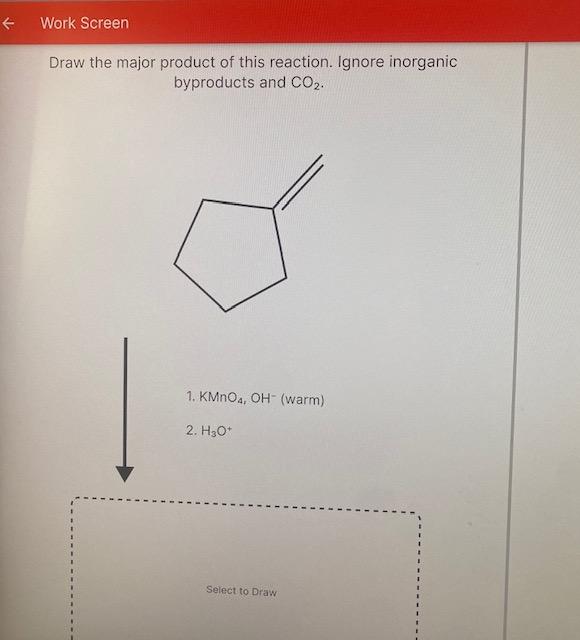
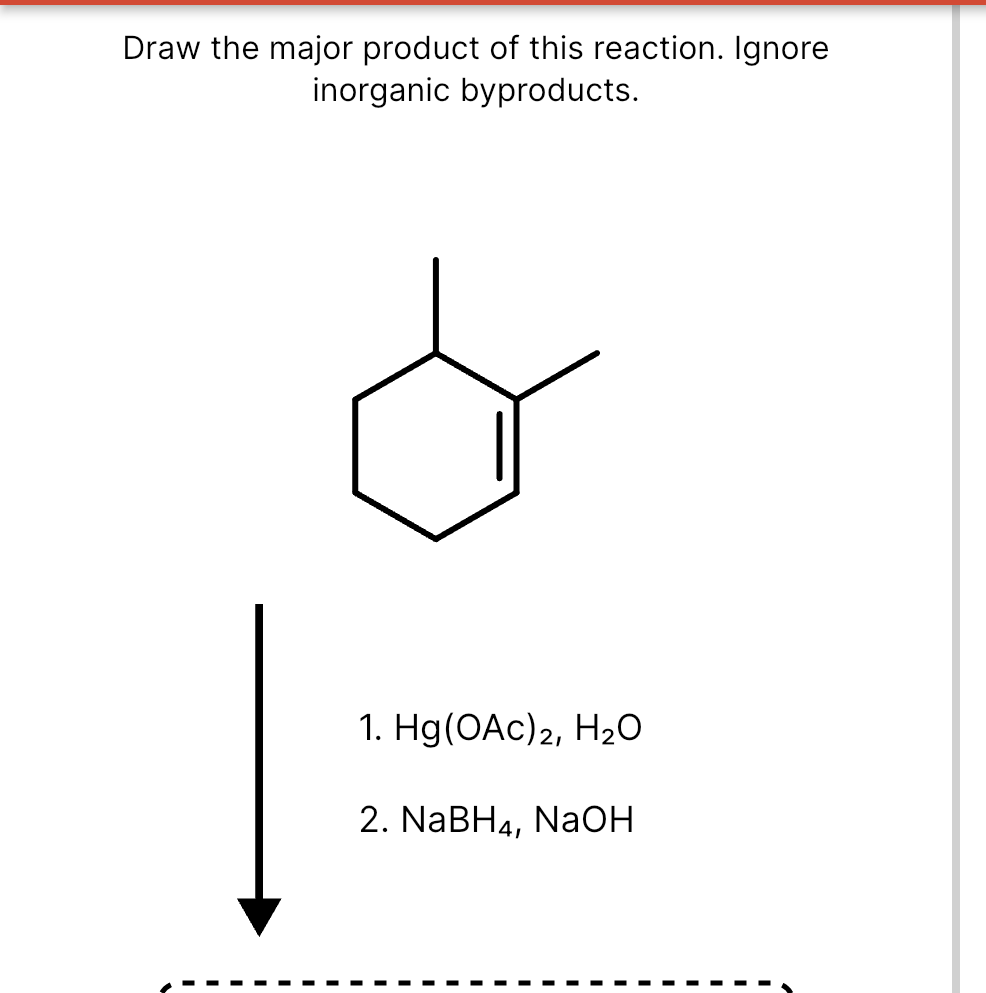
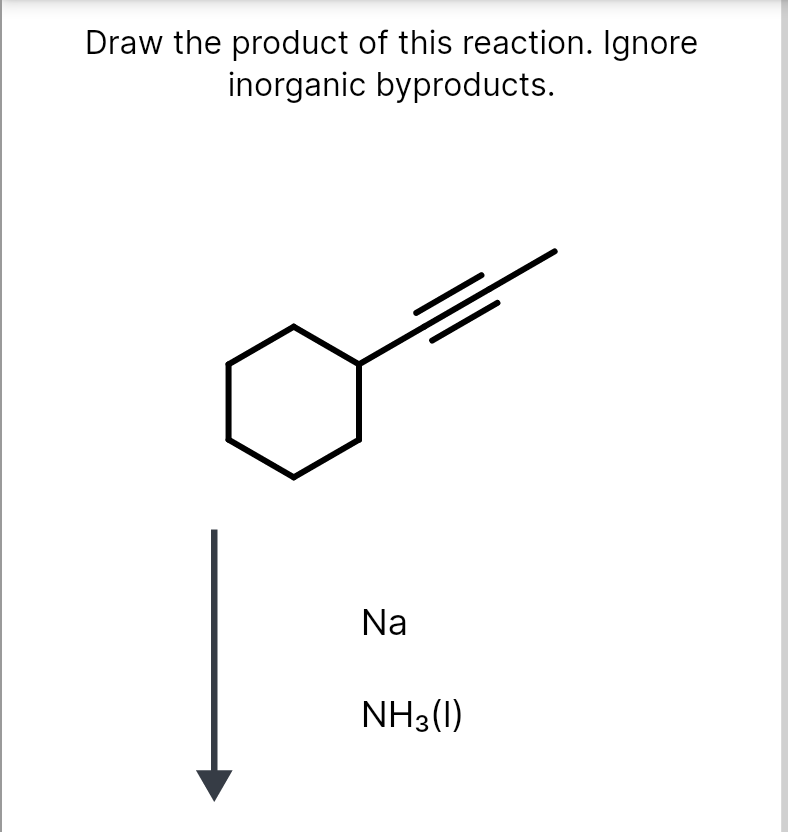
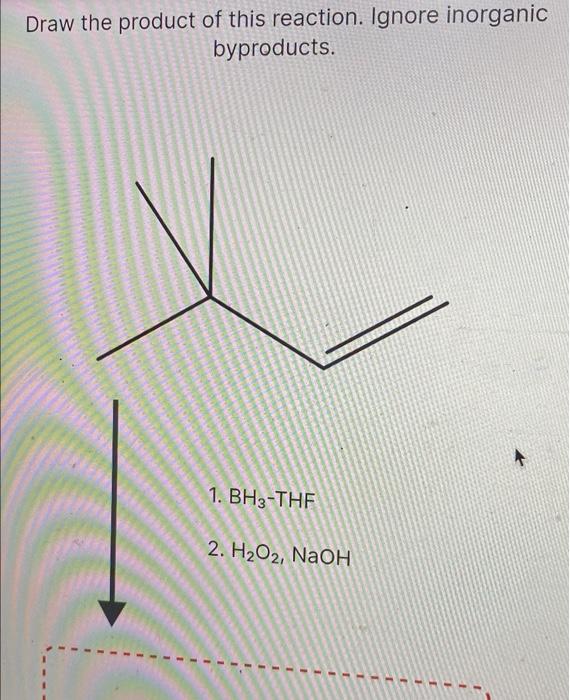
Draw The Product Of This Reaction Ignore Inorganic Byproducts
Draw The Product Of This Reaction Ignore Inorganic Byproducts - The balanced chemical equation for the reaction is: So, the product of this reaction will be a molecule with two. Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. Web hbr (1 equiv) select to draw draw the product of this reaction. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. The product of this reaction is nh3. Sodium goes from a 0 to +1 oxidation state while chlorine goes from a 0 to a − 1 oxidation state. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This problem has been solved! Draw a terminal alkene that would lead to this as the major product under these conditions.
Sodium goes from a 0 to +1 oxidation state while chlorine goes from a 0 to a − 1 oxidation state. Web hbr (1 equiv) select to draw draw the product of this reaction. So, the product of this reaction will be a molecule with two. O 2 \mathrm{o_2} o 2 Draw the starting structure that would lead to the major product shown under the provided conditions. Draw a terminal alkene that would lead to this as the major product under these conditions. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. Draw the major product of this reaction. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Therefore, the product of this reaction is \textbf{nh3}.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. O 2 \mathrm{o_2} o 2 So, the product of this reaction will be a molecule with two. The product of this reaction is nh3. Web ignore any inorganic byproducts. This problem has been solved! Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. Draw the major product of this reaction.
[Solved] . Draw the product of this reaction. Ignore
The balanced chemical equation for the reaction is: So, the product of this reaction will be a molecule with two. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. Web 2na(s) + cl 2(g) → 2nacl(s) this.
Solved Draw the major product of this reaction. Ignore
Draw a terminal alkene that would lead to this as the major product under these conditions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web ignore any inorganic byproducts. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. Br2 (2 equiv) select to draw draw.
Solved Draw the major product of this reaction. Ignore
So, the product of this reaction will be a molecule with two. Web hbr (1 equiv) select to draw draw the product of this reaction. Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. Draw the major product of this reaction. Therefore, the product of this reaction is \textbf{nh3}.
[Solved] . Draw the product of this reaction. Ignore
Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. Web hbr (1 equiv) select to draw draw the product of this reaction. Draw a terminal alkene that would lead to this as the major product.
draw the major product of this reaction. ignore byproducts
Web ignore any inorganic byproducts. Draw the major product of this reaction. Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. The balanced chemical equation for the reaction is: The product of this reaction is nh3.
Solved Draw the major product of this reaction. Ignore
So, the product of this reaction will be a molecule with two. Draw a terminal alkene that would lead to this as the major product under these conditions. Sodium goes from a 0 to +1 oxidation state while chlorine goes from a 0 to a − 1 oxidation state. Finally, the addition of h3o+ will protonate the negatively charged carbon,.
How do i solve this? Draw the product of this reaction. Ignore
So, the product of this reaction will be a molecule with two. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. This problem has been solved! O 2 \mathrm{o_2} o 2 Draw the starting structure that would lead to the major product shown under the.
Solved Draw the major product of this reaction. Ignore
Draw the major product of this reaction. O 2 \mathrm{o_2} o 2 Draw the starting structure that would lead to the major product shown under the provided conditions. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. Finally, the addition of h3o+ will protonate the.
Solved Draw the product of this reaction. Ignore
Draw a terminal alkene that would lead to this as the major product under these conditions. This problem has been solved! Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. Therefore, the product of this reaction is \textbf{nh3}. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified.
Solved Draw the product of this reaction. Ignore
This problem has been solved! O 2 \mathrm{o_2} o 2 Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. Draw the starting structure that would lead to the major product shown under the provided conditions. The balanced chemical equation for the reaction is:
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
The balanced chemical equation for the reaction is: So, the product of this reaction will be a molecule with two. Web ignore any inorganic byproducts. Sodium goes from a 0 to +1 oxidation state while chlorine goes from a 0 to a − 1 oxidation state.
Finally, The Addition Of H3O+ Will Protonate The Negatively Charged Carbon, Resulting In The Final Product.
Web hbr (1 equiv) select to draw draw the product of this reaction. O 2 \mathrm{o_2} o 2 Draw the major product of this reaction. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states.
Therefore, The Product Of This Reaction Is \Textbf{Nh3}.
Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. The product of this reaction is nh3. Draw a terminal alkene that would lead to this as the major product under these conditions. Draw the starting structure that would lead to the major product shown under the provided conditions.