Draw The Products Of The Following Acidbase Reaction
Draw The Products Of The Following Acidbase Reaction - Recognize if an acid or base is strong or weak. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Estimate the ph of each solution given below based on hydronium or hydroxide concentration without using a calculator. Then, in parts 2 and 3 predict which side of this reaction is favored at equilibrium and to what extent. (ii) [h3o+] = 3.5 × 10−2 m. Or carbonate, co 32− ). (i) [ho−] = 2.4 × 10−9 m. Be sure to answer all parts. However, if there is no obvious acid (or base) as in this example, how do you determine. If h + is the acid as in previous examples, it is rather easy to predict how the reaction will proceed.
Using our pk, table, write all relevant pk, values (i.e. Interactive 3d display mode draw the products on the canvas by choosing buttons from the tools (for bonds), atoms. Determine the exact ph by using a calculator. Label the acid, base, conjugate acid, and conjugate base: This problem has been solved! However, if there is no obvious acid (or base) as in this example, how do you determine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (i) [ho−] = 2.4 × 10−9 m. 2 views 3 days ago. Hcl (ch3)2c=oh+ ch 3oh +.
Be sure to answer all parts. For the following reaction draw the flipped chair and draw the elimination product of the reaction. Or carbonate, co 32− ). Organic molecules in buffered solution: Organic acid and base reaction. Web chemistry questions and answers. Or acetic acid, ch 3 co 2 h) or electrically charged (ions, such as ammonium, nh 4+; However, if there is no obvious acid (or base) as in this example, how do you determine. Using our pk, table, write all relevant pk, values (i.e. Do not draw lone pairs in your answers.
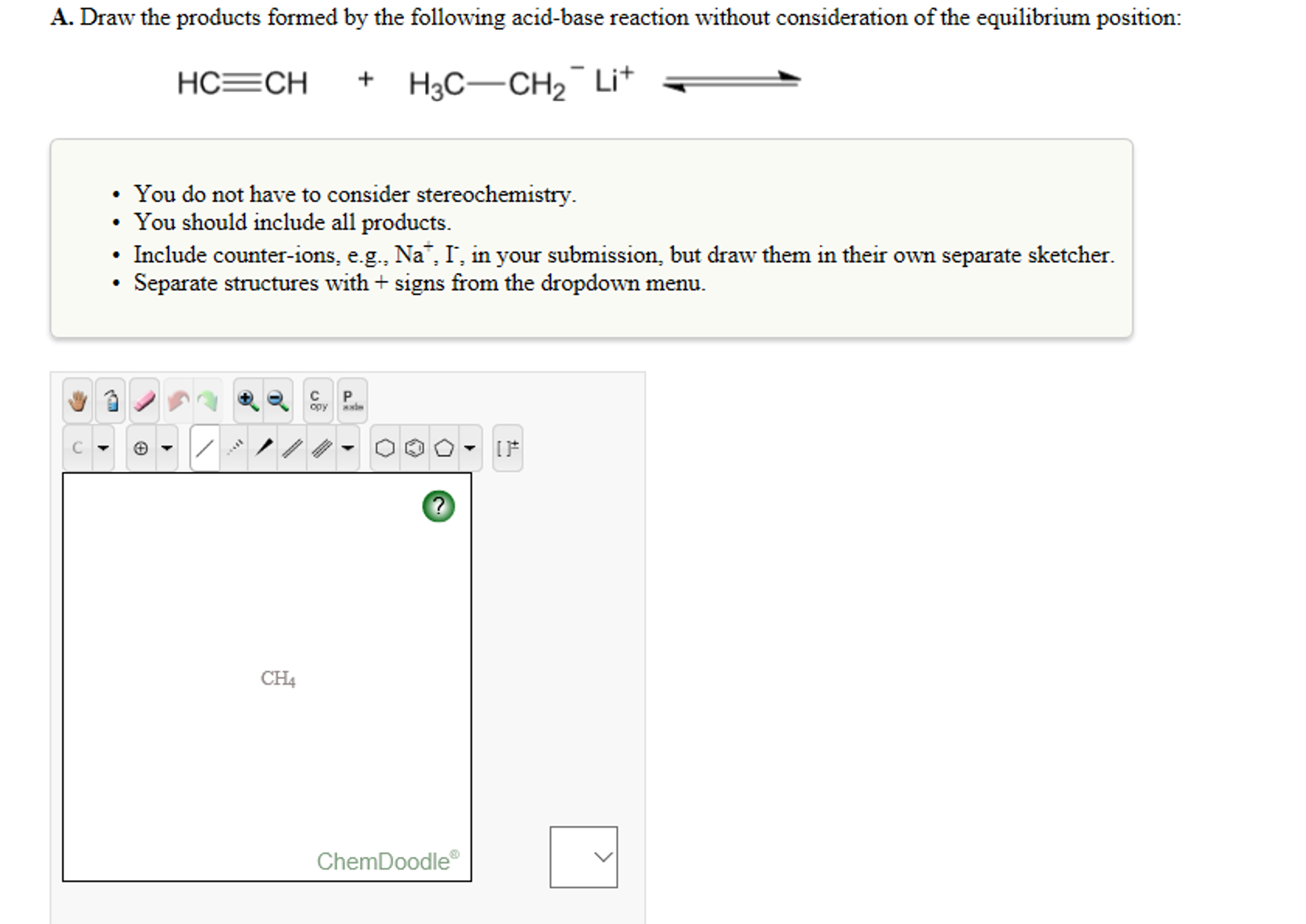
Solved draw the products formed by the following acidbase
Estimate the ph of each solution given below based on hydronium or hydroxide concentration without using a calculator. Be sure to answer all parts. Using our pk, table, write all relevant pk, values (i.e. This content is for registered users only. And then now this oxygen we have three lone pairs of electrons around it which gives this.
Draw The Organic Product(s) Of The Following Reaction. Hcl Ether The
Determine the exact ph by using a calculator. Predict and draw the products of following reaction and use curved arrow to show the mechanism. Na oh + na,coz + na hco.” edit structure. 2 views 3 days ago. Web chemistry questions and answers.
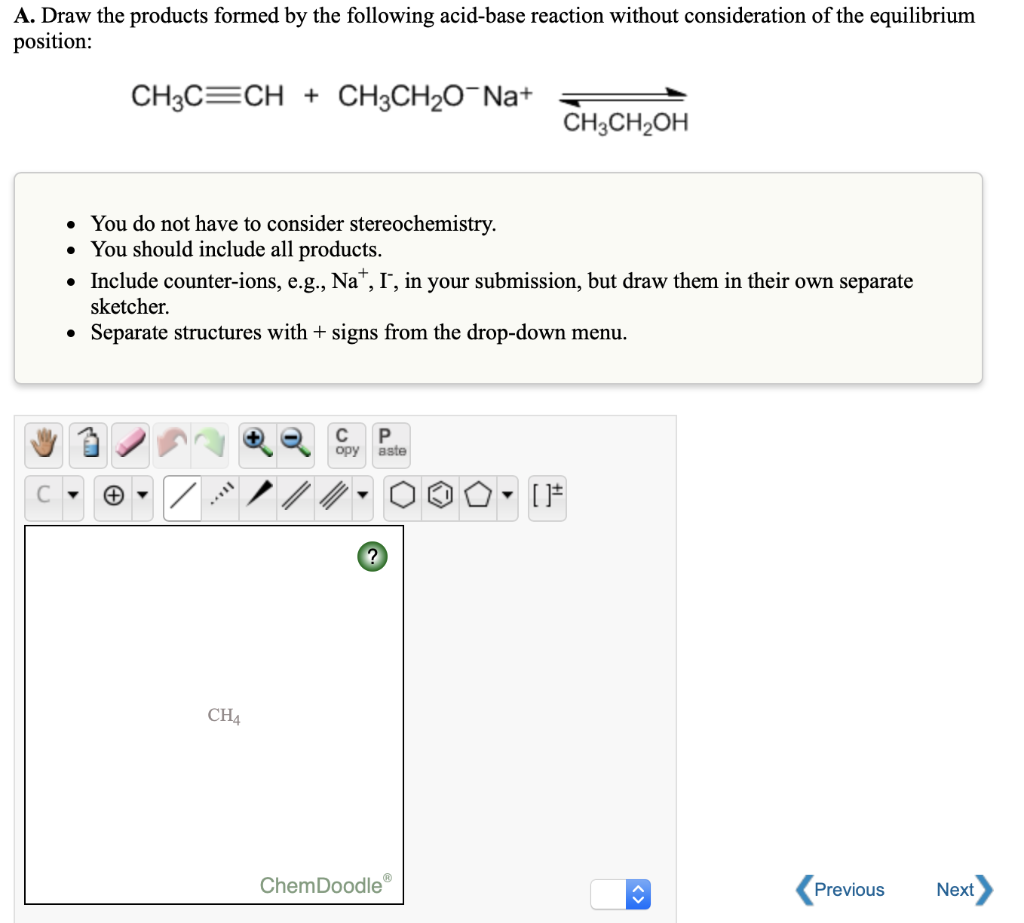
Solved A. Draw the products formed by the following
Web draw the products of the following acid/base reactions, depict the proton exchange with mechanistic arrows, and circle the proper reaction arrow to indicate if reactants or products (or neither) are favored at equilibrium. (6 points) h br draw in the box flipped chair + кок bulky base 6 Organic acid and base reaction. Or acetic acid, ch 3 co.
Draw the organic product of the Lewis acidbase reaction shown below
Or acetic acid, ch 3 co 2 h) or electrically charged (ions, such as ammonium, nh 4+; O products o reactants o neither. Organic acid and base reaction. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; (ii) [h3o+] = 3.5 × 10−2 m.
OneClass Draw the products of the following acid/base reactions
However, if there is no obvious acid (or base) as in this example, how do you determine. Using our pk, table, write all relevant pk, values (i.e. Or acetic acid, ch 3 co 2 h) or electrically charged (ions, such as ammonium, nh 4+; Predict and draw the products of following reaction and use curved arrow to show the mechanism..
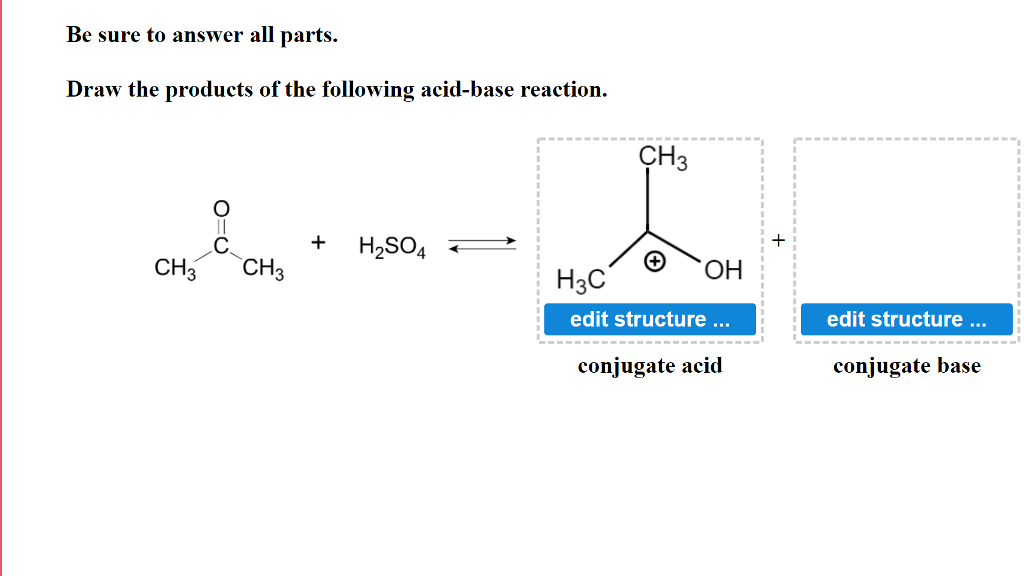
Solved Be sure to answer all parts. Draw the products of the
Using our pk, table, write all relevant pk, values (i.e. (i) [ho−] = 2.4 × 10−9 m. Organic molecules in buffered solution: So on the left we would have our carbon double bonded to our oxygen. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
[Solved] Draw the organic product of the Lewis acidbase
Naoh + hcl → nacl + h₂o naoh + ch₃cooh → ch₃coona + h₂o Hcl (ch3)2c=oh+ ch 3oh +. Or carbonate, co 32− ). Identify the acid and base in chemical reaction. This content is for registered users only.
Solved Draw the products of the following acidbase
Include all lone pairs and nonzero formal charges. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; So on the left we would have our carbon double bonded to our oxygen. (iii) [h3o+] = 5.7 × 10−4 m. Estimate the ph of each solution given below based on hydronium or hydroxide concentration.
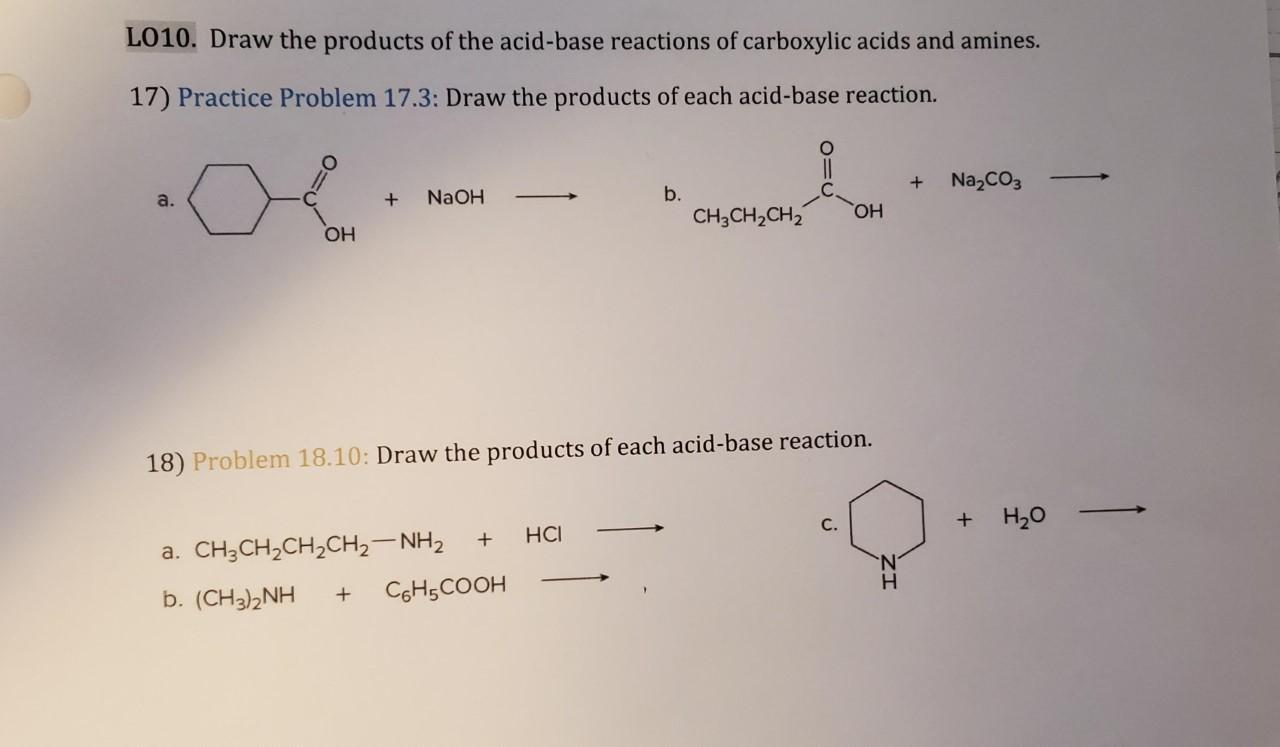
Solved L010. Draw the products of the acidbase reactions of
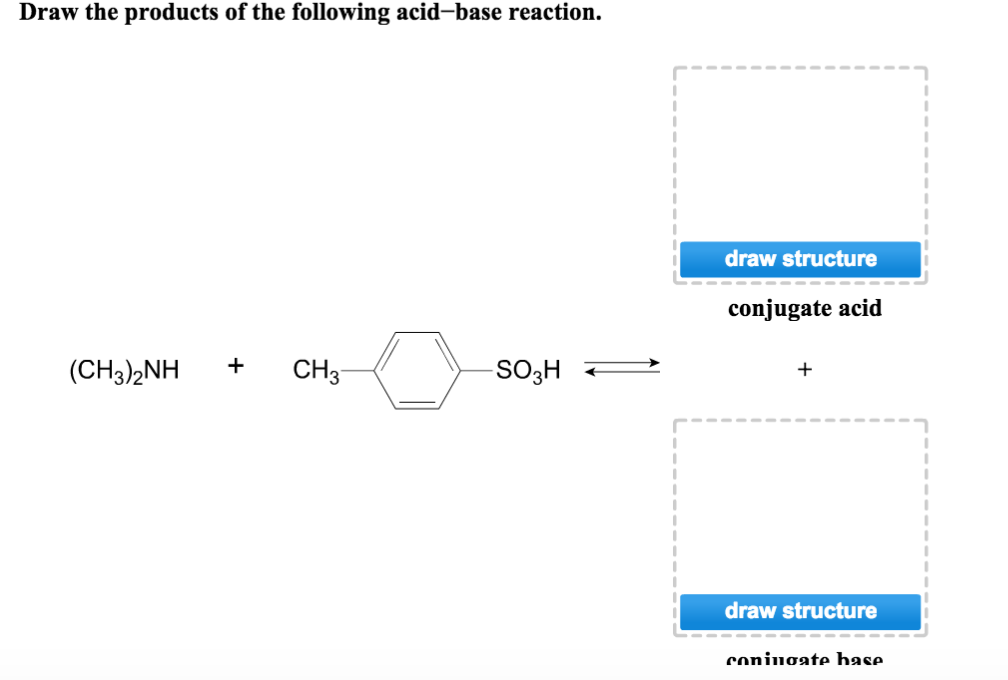
(ii) [h3o+] = 3.5 × 10−2 m. This problem has been solved! For the two reactants if available and the conjugate acid). 2 views 3 days ago. (ch3)2nh + ch3 so3h + draw structure.
[Solved] draw the product/s of the following acidbase reaction
If h + is the acid as in previous examples, it is rather easy to predict how the reaction will proceed. Or carbonate, co 32− ). Be sure to answer all parts. Naoh + hcl → nacl + h₂o naoh + ch₃cooh → ch₃coona + h₂o So on the left we would have our carbon double bonded to our oxygen.
Be Sure To Answer All Parts.
However, if there is no obvious acid (or base) as in this example, how do you determine. Organic molecules in buffered solution: (iii) [h3o+] = 5.7 × 10−4 m. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Organic Acid And Base Reaction.
So on the left we would have our carbon double bonded to our oxygen. Or carbonate, co 32− ). Label the acid, base, conjugate acid, and conjugate base: Be sure to answer all parts.
(Ii) [H3O+] = 3.5 × 10−2 M.
This problem has been solved! Hcl (ch3)2c=oh+ ch 3oh +. Na oh + na,coz + na hco.” edit structure. For the following reaction draw the flipped chair and draw the elimination product of the reaction.
Determine The Exact Ph By Using A Calculator.
For the two reactants if available and the conjugate acid). This problem has been solved! And then now this oxygen we have three lone pairs of electrons around it which gives this. This content is for registered users only.
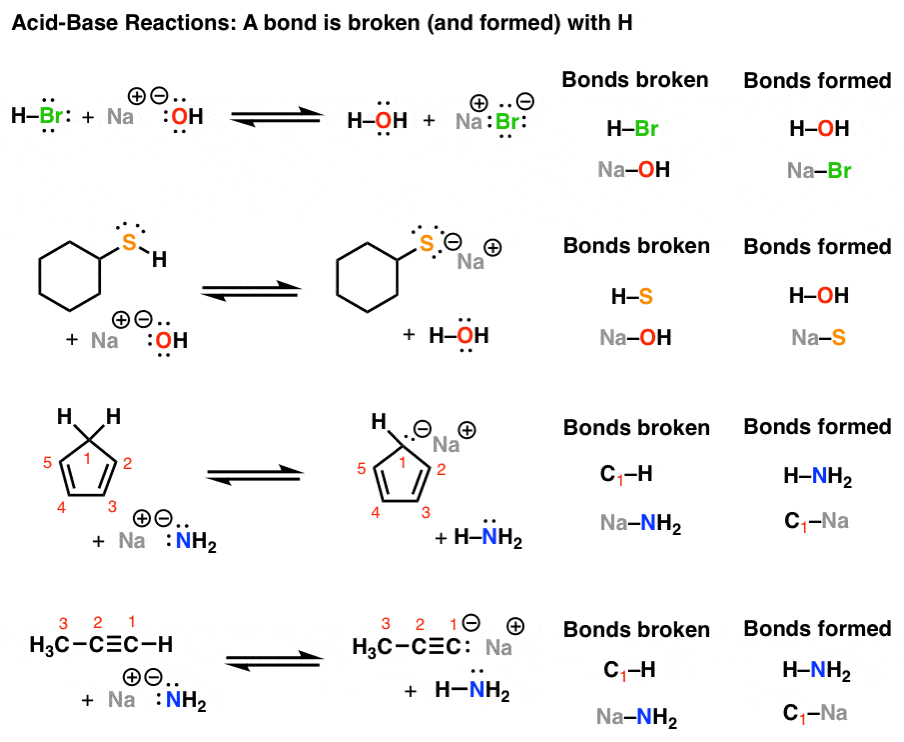

![[Solved] Draw the organic product of the Lewis acidbase](https://media.cheggcdn.com/media/fe4/fe4d6aa3-e002-4a47-bca6-90d53377b20e/phpMzExp1)