Drawing Covalent Bonds Worksheet
Drawing Covalent Bonds Worksheet - Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. Web ionic bond covalent bond. How are ionic bonds and covalent bonds different? Describe the relationship between the length of a bond and the strength of that bond. Single, double, and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. Worksheets to support the students in working out and drawing covalent bonding in simple molecules. Review of ws#1 and ws#2. Show the work and the final answer remember: Draw the lewis diagram for the covalent bond in the hcl molecule. Identify the type(s) of bond(s) found in the following molecules:
Water is an example of a molecule with this type of bond. Draw the lewis diagram for the covalent bond in the h 2 molecule. What is the difference between a molecule and a formula unit? A) hydrogen (h 2) b) bromine (br 2) c) water (h 2 o) d) ammonia (nh 3) 2. The bonds in f 2. Review of ws#1 and ws#2. Web chapters 6 and 7 practice worksheet: Included are aspects of both ionic bonding and covalent bonding. Looking to test how well your students understand chemical bonding? Then this covalent bonding worksheet with answer key is a perfect fit for your classroom!
Then this covalent bonding worksheet with answer key is a perfect fit for your classroom! Circle the unpaired electrons that will be shared between the elements. Draw the lewis diagram for the covalent bond in the hcl molecule. Show the work and the final answer remember: Web covalent and ionic compounds. (recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. Web chapters 6 and 7 practice worksheet: Web this worksheet clearly explains how to draw dot and cross diagrams for covalent compounds, using cl2 as an example. Review of ws#1 and ws#2. Draw the lewis diagram for the covalent bond in the h 2 molecule.
Drawing Covalent Bonds Worksheet Worksheets For Kindergarten
Review of ws#1 and ws#2. Draw the single bonds below. (recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. A) hydrogen (h 2) b) bromine (br 2) c) water (h 2 o) d) ammonia (nh 3) 2. Web this worksheet clearly explains how to draw dot and cross diagrams.
Covalent Bonding The Science and Maths Zone
Extension draw a cluster diagram for each type of bond. Determine the total number of valence electrons in the molecule or ion. Determine if it is an ionic bond or a covalent bond. Draw the lewis diagram for the covalent bond in the br 2 molecule. Describe the relationship between the length of a bond and the strength of that.
Covalent Bonding Practice Worksheets
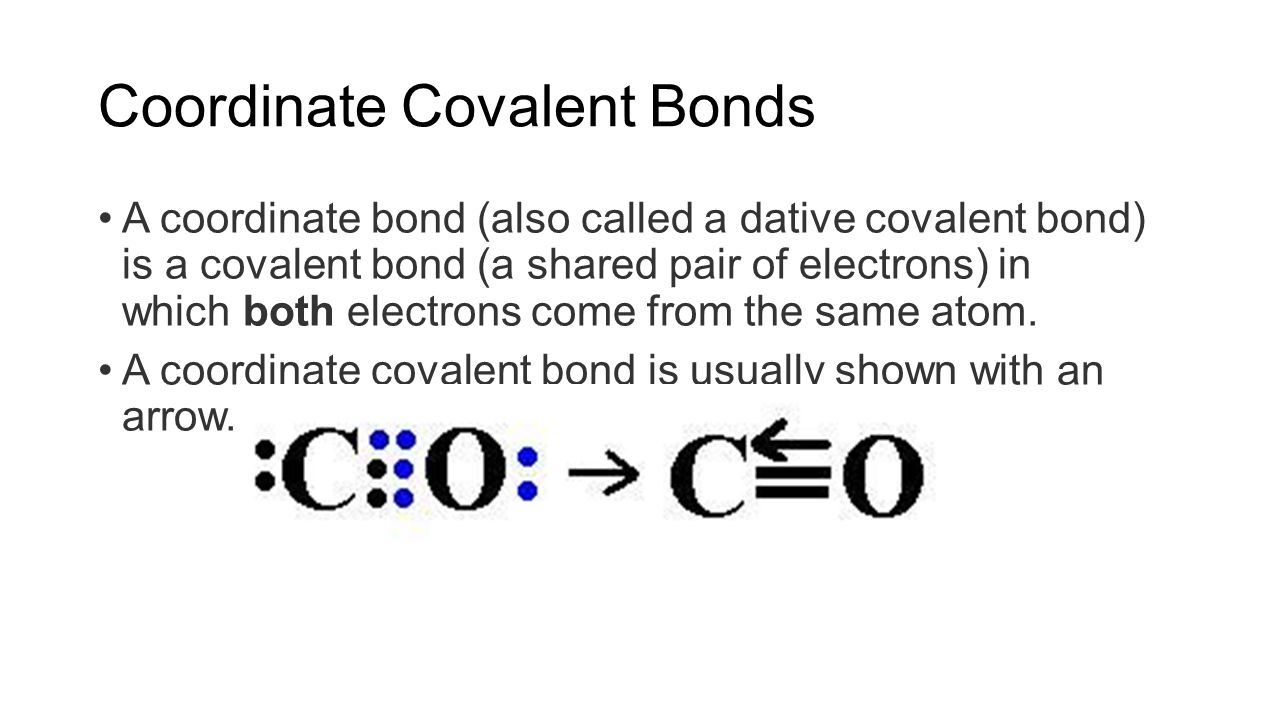
Covalent bonds and molecular structure. Water is an example of a molecule with this type of bond. 2 sample questions showing how to properly draw covalent bond diagrams. The bonds in h 2 o. Web define covalent bond.
Covalent bond worksheet Teaching Resources
• types of elements involved in covalent bonds. Web the covalent bonding worksheet covers the following topics: Draw the single bonds below. Draw the lewis diagram for the covalent bond in the hcl molecule. Water is an example of a molecule with this type of bond.
drawing single covalent bonds worksheet vansblackandwhitelowtops
• interpreting diagrams representing covalent bonds. Determine if it is an ionic bond or a covalent bond. Worksheets to support the students in working out and drawing covalent bonding in simple molecules. Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. Web sharing 1 pair of electrons creates a single.
How is a covalent bond formed
Ionic bonds are formed between a metal. Add together the valence electrons from each atom. Covalent bonds form between two nonmetals that share electrons. Arrange the atoms to show specific bonds. Extension draw a cluster diagram for each type of bond.
Chemical Bonds Ionic Bonds Worksheet
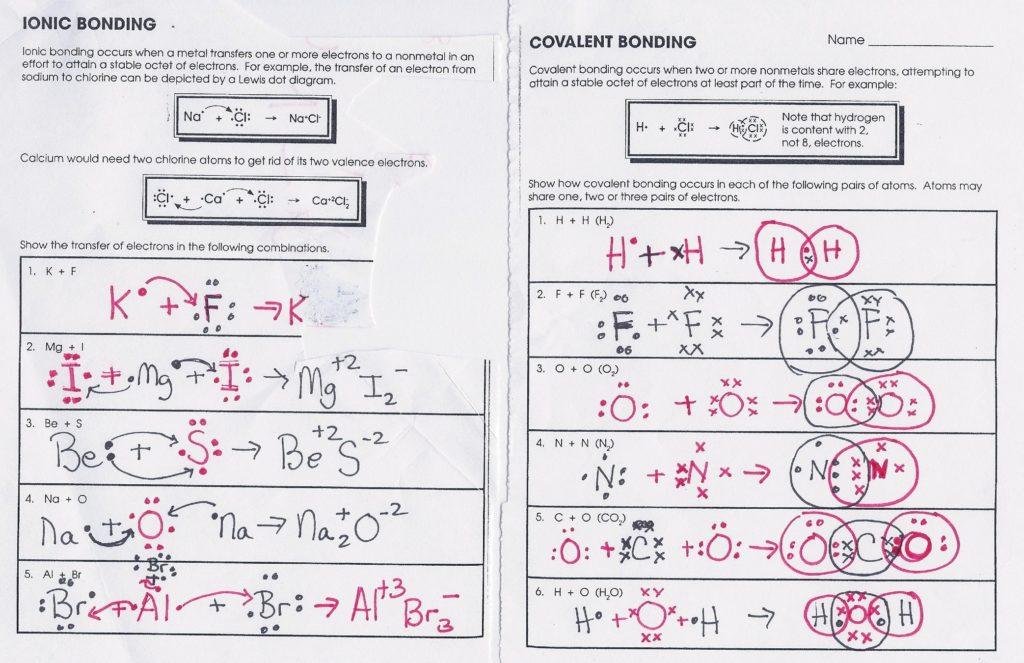
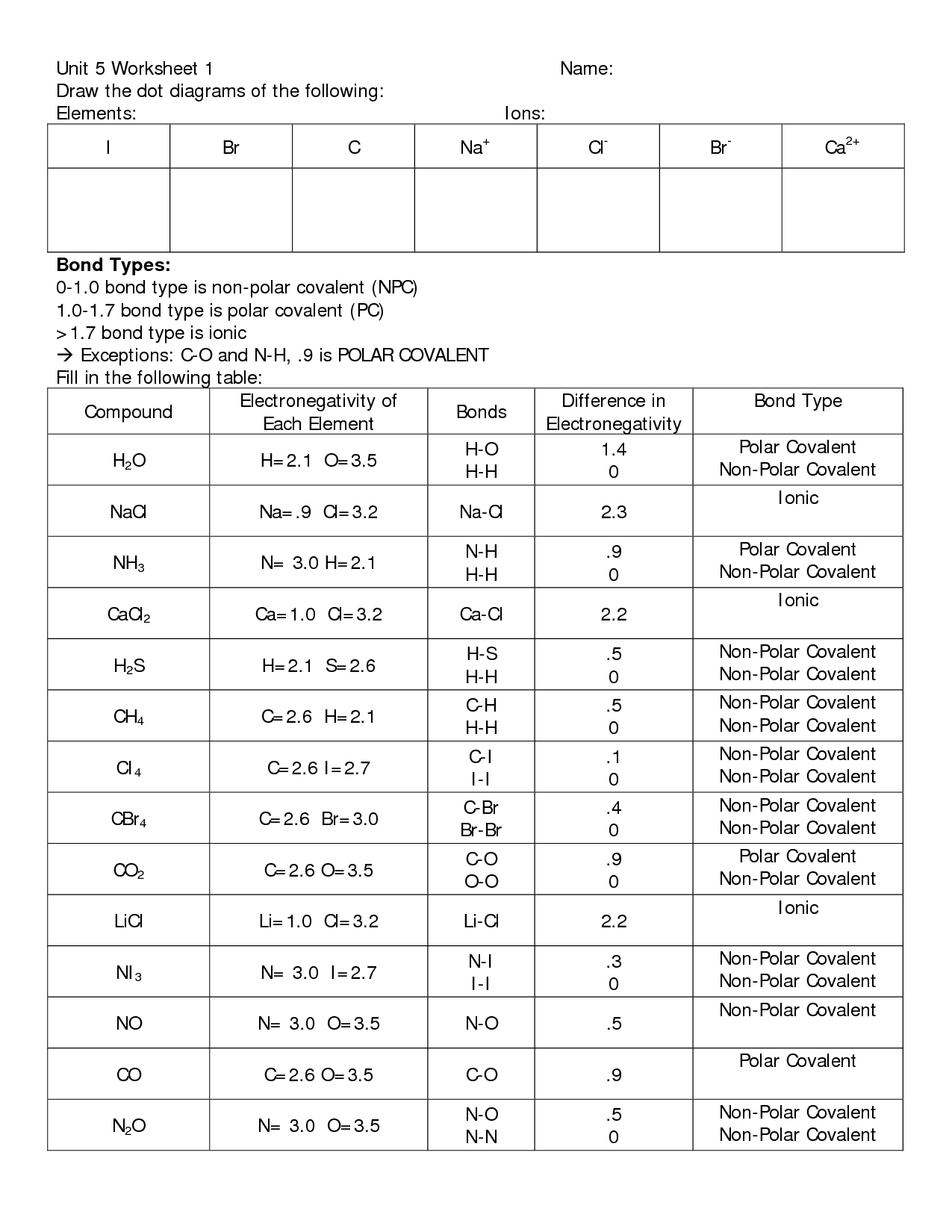
Sharing electrons in covalent bonds; Co 2 is nonpolar because the two polar bonds are equal and opposite so cancel out h 2o is polar because the bonds are not. Draw the lewis diagram for the covalent bond in the hcl molecule. Included are aspects of both ionic bonding and covalent bonding. Draw the electron dot diagrams for each element.**
Drawing Covalent Bonds Worksheet Martin Lindelof
Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. Identify the type(s) of bond(s) found in the following molecules: The bonds in h 2 o. What is the difference between a molecule and a formula unit? Web the bonds between the carbon atom and the hydrogen atoms in the compound.
14 Lewis Structures Covalent Bonding Worksheet /
Interpreting diagrams representing covalent bonds; This worksheet challenges your students to match up the substances listed on the left with the kind of bond they form. Ionic bonds are formed between a metal. • types of elements involved in covalent bonds. A) hydrogen (h 2) b) bromine (br 2) c) water (h 2 o) d) ammonia (nh 3) 2.
Covalent Bonding Worksheet Pdf
Show the work and the final answer remember: Web the bonds between the carbon atom and the hydrogen atoms in the compound methane ch 4 are examples of covalent bonds between two different elements. • types of elements involved in covalent bonds. Circle the unpaired electrons that will be shared between the elements. Draw the single bonds below.
Co 2 Is Nonpolar Because The Two Polar Bonds Are Equal And Opposite So Cancel Out H 2O Is Polar Because The Bonds Are Not.
Ionic bonds are formed between a metal. Web chapters 6 and 7 practice worksheet: Web relates to covalent bonding. Web the covalent bonding worksheet covers the following topics:
Draw The Electron Dot Diagrams For Each Element.**
Draw a lewis dot diagram for each element listed. This is a pretty length chapter that goes into the specifics of how elements bond with each other. Most of the time is spent on covalent bonding, with more advanced topics such as molecular geometry. Web ionic bond covalent bond.
Single, Double, And Triple Covalent Bonds Depend On The Number Of Pairs Of Electrons Shared Between Two Atoms.
Identify the type(s) of bond(s) found in the following molecules: Sharing electrons in covalent bonds; Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. • types of elements involved in covalent bonds.
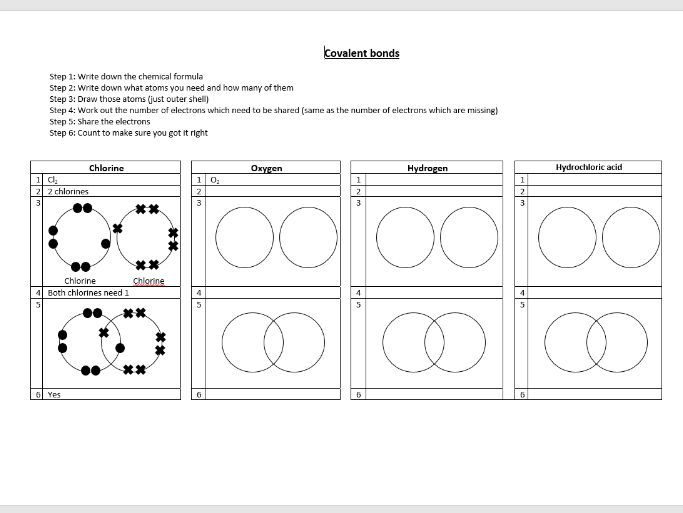
Web A Worksheet For Covalent Bonding With Some Bond Drawing Practice And A Space To Work On Different Types Of Covalent Structures (Small And Giant, Including Allotropes Of Carbon, Designed Around The Specification For Aqa Gcse Chemistry/Combined Science In The Bonding Topic.
Web structured worksheet guiding students on the construction of dot and cross diagrams to represent the covalent bonding in simple covalent molecules.resource includes:+ step by step guide to drawing dot and cross diagrams+ worked examples for single covalent bonds (fluorine, f2) and multiple covalent bonds (carbon dioxide, co2)+ structured. Determine the total number of valence electrons in the molecule or ion. The bonds in k 2 o. Be able to recognize whether the type of bond between two atoms is covalent, polar covalent or ionic.