Drawing Molecular Geometry
Drawing Molecular Geometry - Explore molecule shapes by building molecules in 3d! Learn how molecule shapes change with different bonds and electron pairs. Vespr stands for valence shell electron pair repulsion. Web here's some of the guidelines for drawing dot structures. Web the following procedure uses vsepr theory to determine electron group geometry and molecular structures (molecular shape): Explore molecule shapes by building molecules in 3d! Determine the electron geometry from the lewis dot structure. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Find out how a molecule's shape changes as you add atoms to a molecule.
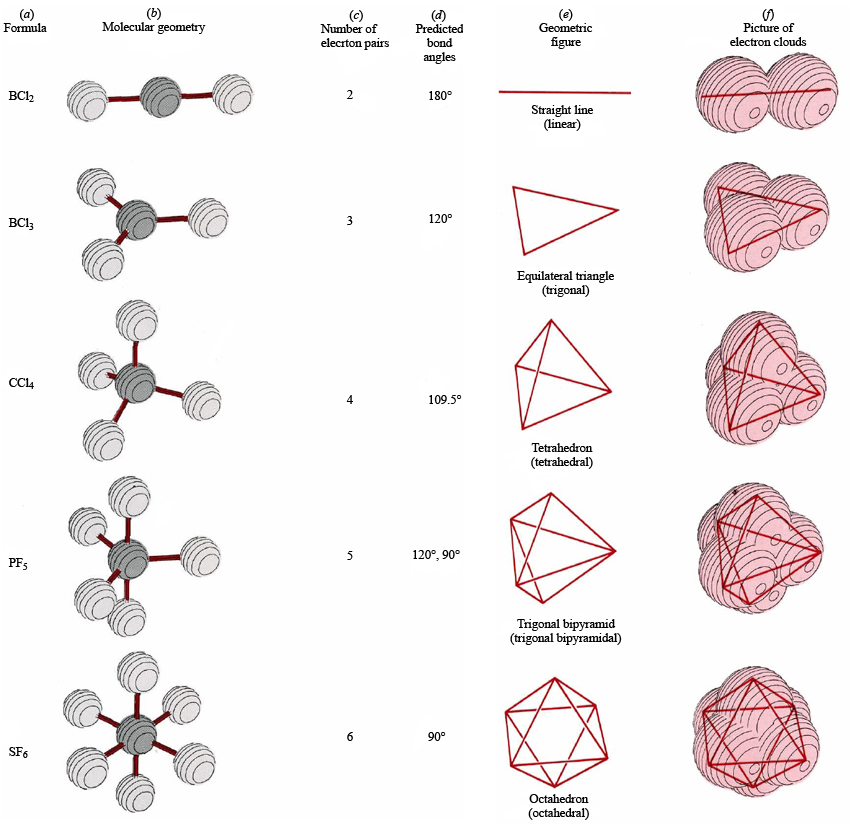
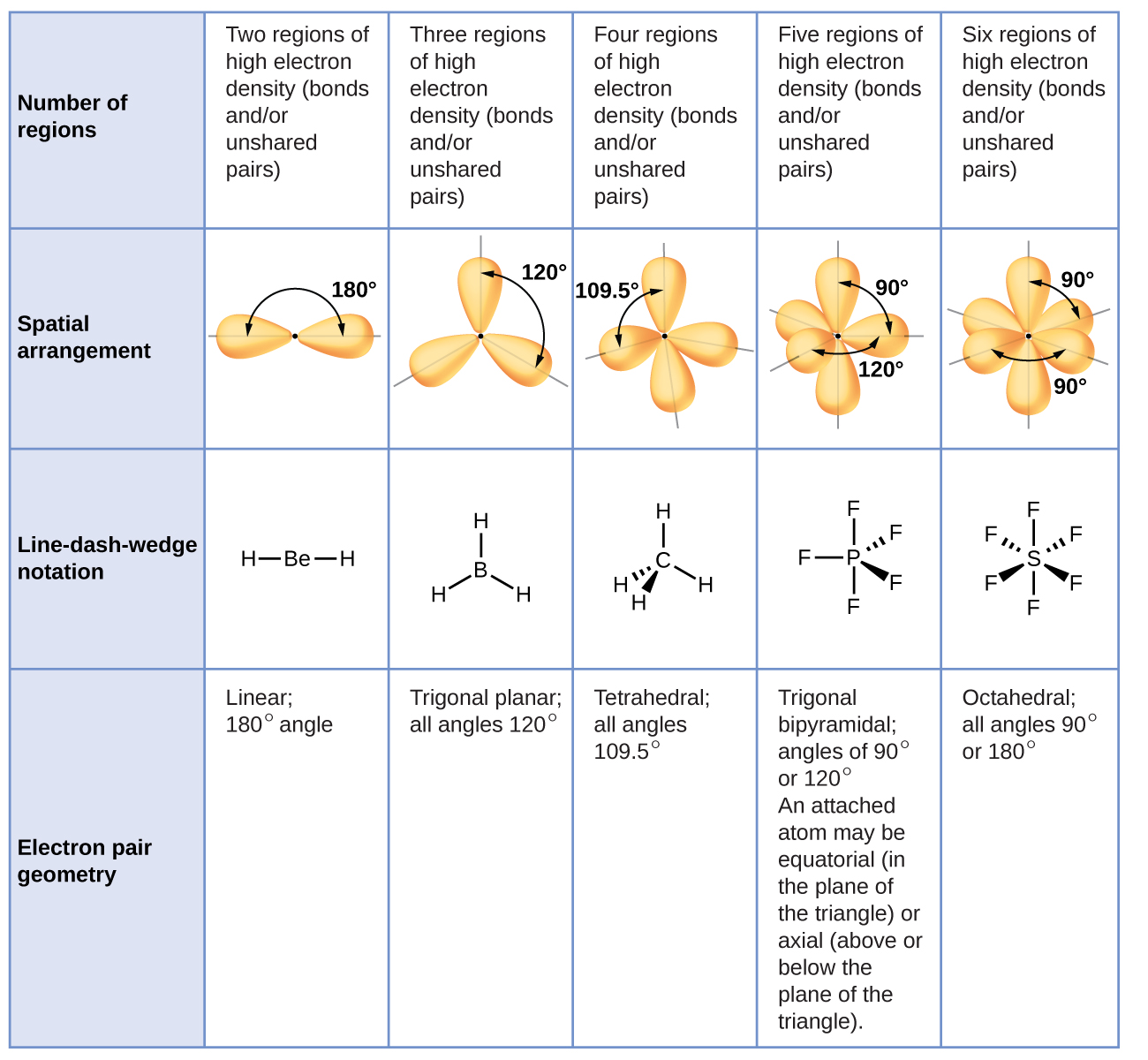
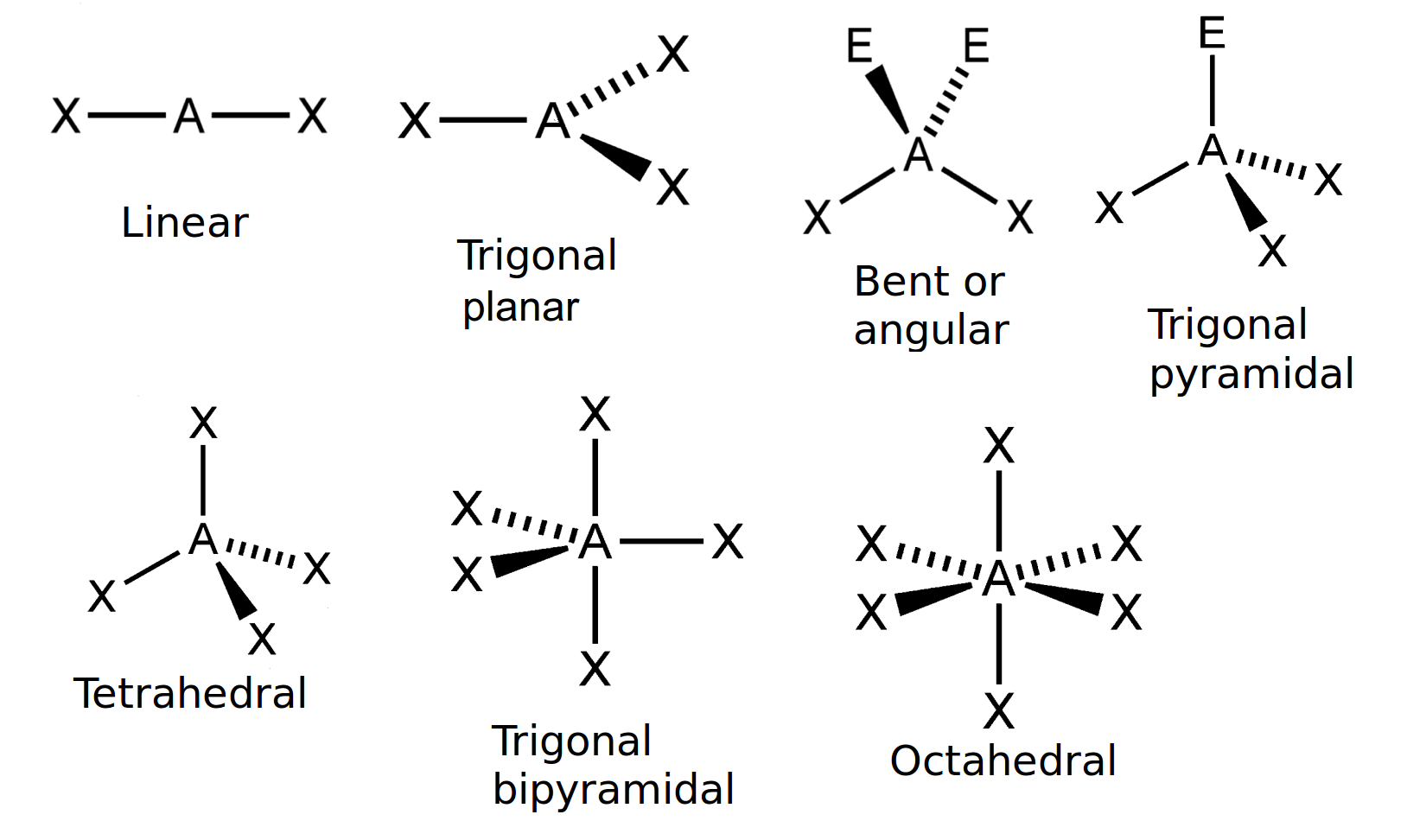
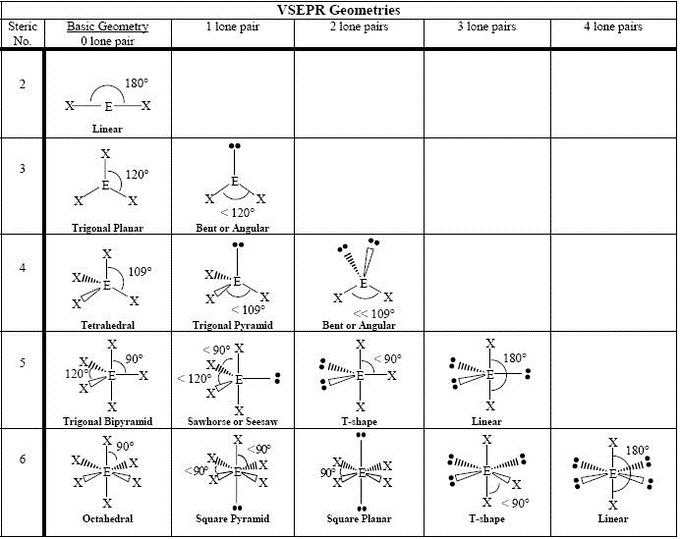
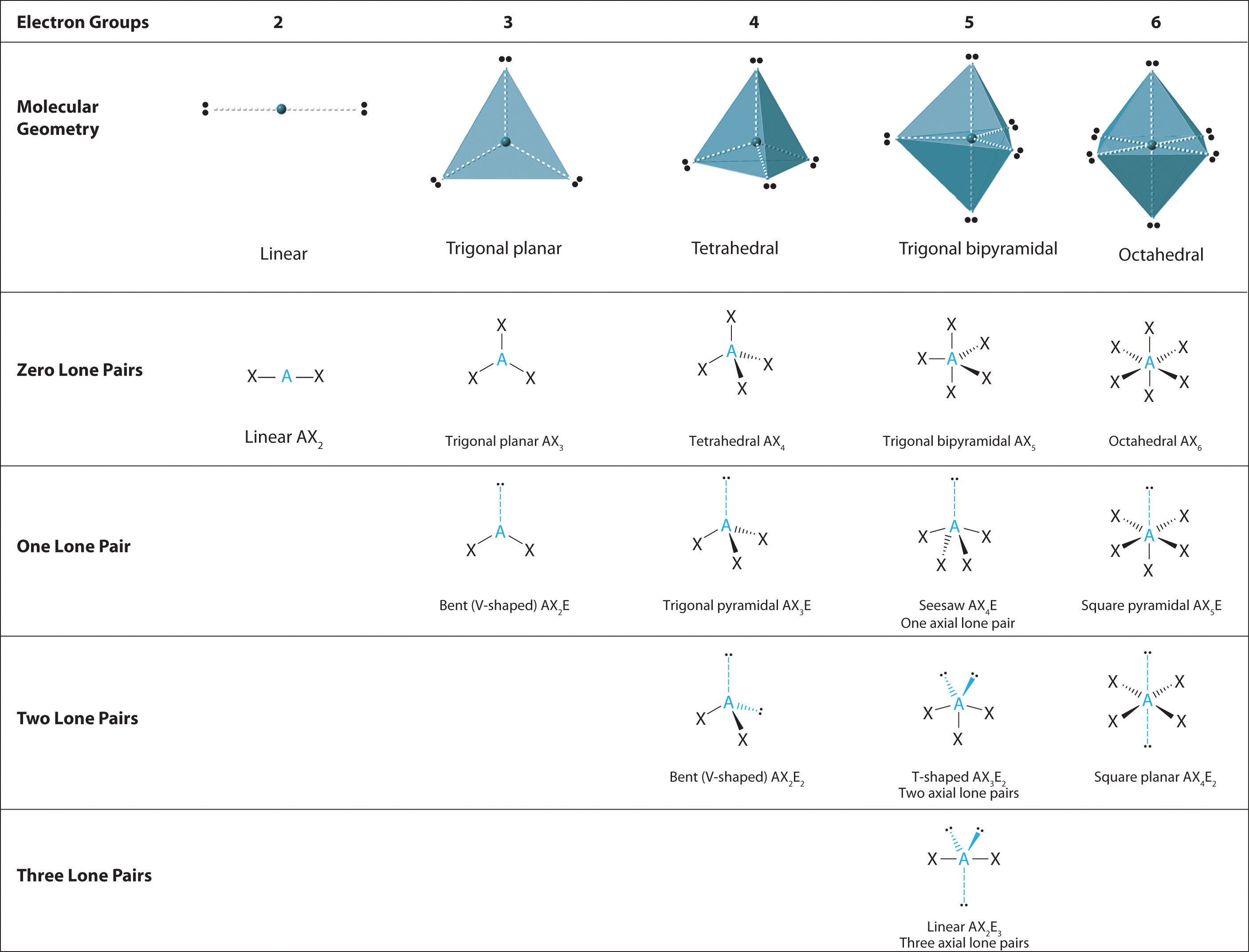
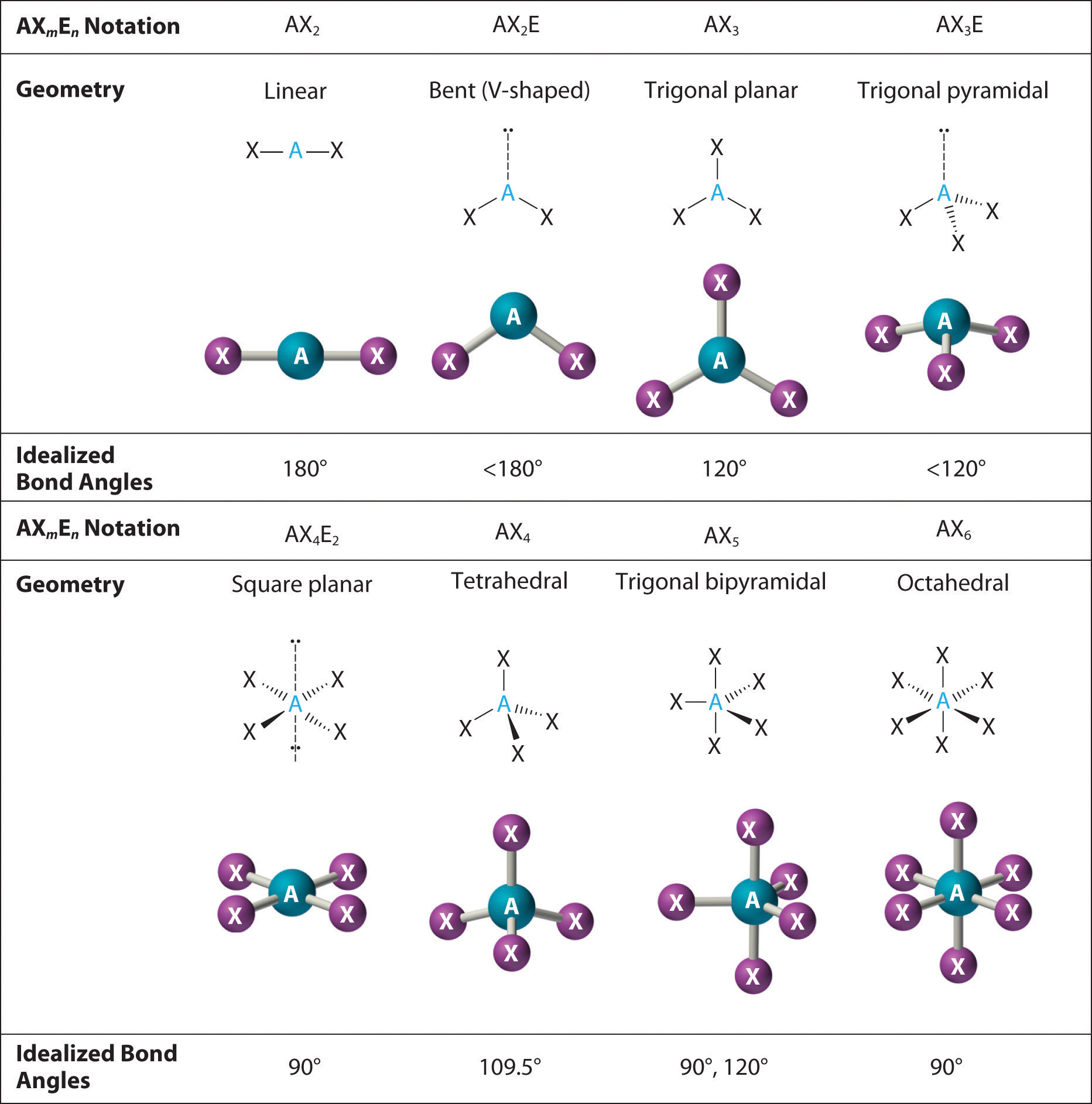
Draw a diagram of a tetrahedral or octahedral molecule. Determine the electron geometry from the lewis dot structure. It applies a theory called vespr for short. Determine the lewis dot structure of the compound. Web there is a three step approach to determining the geometry of a molecule. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Web we recommend using the latest version of chrome, firefox, safari, or edge. If you are developer and would like to learn how to install this sketcher, please refer to the sketchercanvas documentation. Web molecular geometry is a way of describing the shapes of molecules. The table of molecular geometries can be found in the first figure.
Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Web explain the distinction between coordination geometry and molecular geometry, and provide an illustration based on the structure of water or ammonia. These problems are for you alone and will not be graded. Compare your models with real molecules. Determine the lewis dot structure of the compound. Explore molecule shapes by building molecules in 3d! How does molecule shape change with different numbers of bonds and electron pairs? Explore molecule shapes by building molecules in 3d! They’re good at helping you get acquainted with the placement of electrons around atoms, helping to visualize molecular geometry, and finally to remember the location of the lone pairs. The single molecule sketcher provides a streamlined interface for drawing a single molecular structure.
Molecular Geometry Chemistry Socratic
Web what is the molecular geometry of clf 3? Count the number of electron groups or regions of electron density (lone pairs and bonds) around the central atom. It applies a theory called vespr for short. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Web we recommend using the latest version of.
Molecular Geometry Boundless Chemistry
Web molecular geometry is a way of describing the shapes of molecules. Then, compare the model to real molecules! Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. If you are developer and would like to learn how to install this sketcher, please refer to the sketchercanvas documentation. It includes the general shape of.
7.6 Molecular Structure and Polarity Chemistry
They’re good at helping you get acquainted with the placement of electrons around atoms, helping to visualize molecular geometry, and finally to remember the location of the lone pairs. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules. Web.
H2O Lewis Structure, Molecular Geometry, and Hybridization
Molview consists of two main parts, a structural formula editor and a 3d model viewer. Web we recommend using the latest version of chrome, firefox, safari, or edge. There are essentially three uses for lewis structures. Explore molecule shapes by building molecules in 3d! Web the following procedure uses vsepr theory to determine electron group geometry and molecular structures (molecular.
3.2 Molecular shape Atomic combinations Siyavula
Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Explore molecule shapes by building molecules in 3d! Web we recommend using the latest version of chrome, firefox, safari, or edge. Determine the lewis dot structure of the compound. There are essentially three uses for lewis structures.
Molecular Geometry Introductory Chemistry
The single molecule sketcher provides a streamlined interface for drawing a single molecular structure. The table of molecular geometries can be found in the first figure. Web molecular geometry gives information about the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Web.
ClO4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Explore molecule shapes by building molecules in 3d! Then, compare the model to real molecules! Web we recommend using the latest version of chrome, firefox, safari, or edge. Web molecular geometry gives information about the general shape of the molecule as well as bond lengths,.
9.7 The Shapes of Molecules Chemistry LibreTexts
The table of molecular geometries can be found in the first figure. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters. Web molecular geometry gives information about the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters.
9.7 The Shapes of Molecules Chemistry LibreTexts
Learn how molecule shapes change with different bonds and electron pairs. Web what is the molecular geometry of clf 3? Web build and explore molecules in 3d with this interactive simulation. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. The structural formula editor is surround by three toolbars which contain the tools.
C2H2 Molecular Geometry / Shape and Bond Angles (see description for
Find out how a molecule's shape changes as you add atoms to a molecule. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules. Web what is the molecular geometry of clf 3? Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is.
Explore Molecule Shapes By Building Molecules In 3D!
How does molecule shape change with different numbers of bonds and electron pairs? Web molecular geometry is a way of describing the shapes of molecules. The table of molecular geometries can be found in the first figure. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.
Molview Consists Of Two Main Parts, A Structural Formula Editor And A 3D Model Viewer.
Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Find out by adding single, double or triple bonds and lone pairs to the central atom. Vespr stands for valence shell electron pair repulsion. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules.
Then, Compare The Model To Real Molecules!
Web molecular geometry gives information about the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Web the following procedure uses vsepr theory to determine electron group geometry and molecular structures (molecular shape): Web there is a three step approach to determining the geometry of a molecule. If you are developer and would like to learn how to install this sketcher, please refer to the sketchercanvas documentation.
The First Thing We Would Need To Do Is To Find The Total Number Of Valence Electrons.
Find out how a molecule's shape changes as you add atoms to a molecule. These problems are for you alone and will not be graded. There are essentially three uses for lewis structures. Count the number of electron groups or regions of electron density (lone pairs and bonds) around the central atom.