H20 Drawing
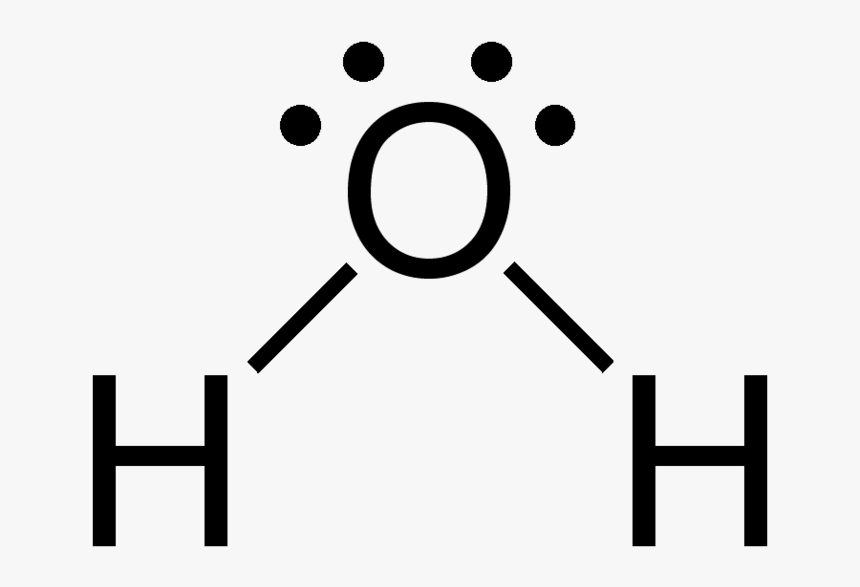
H20 Drawing - You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. On this picture you can see tetrahedral shapes of water, ammonia and methane. Because the water molecule has four electron domains (the two hydrogen. See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. In order to find the total valence electrons in h2o molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. 840 views 4 years ago lewis structure (chemistry) how to draw lewis structure for h2o water lewis structure: Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. Remember that hydrogen only needs two electrons to have a full outer shell. Find the total valence electrons in h2o molecule. Web #1 draw a rough skeleton structure.
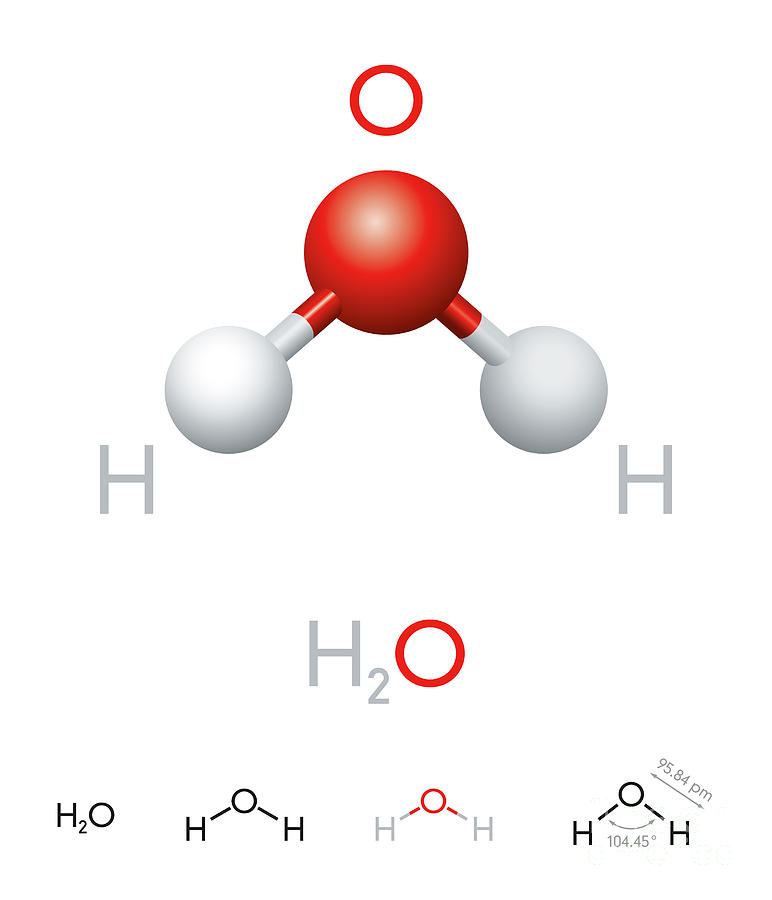
Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. 13k views 4 years ago. Web #1 draw a rough skeleton structure. For h₂o, o must be the central atom. Hence, hydrogen has one valence electron and oxygen has six valence electrons. A common error it to put two oxygen atoms and one hydrogen making ho 2. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: In this article, we will delve into the details of the h2o lewis structure, including how to draw it, its properties, and faqs. How to draw the dot structure for h2o | chemical bonding | success in chemistry. Web explore the molecular structure and geometry of water, one of the most common and important substances on earth.
Web what is the lewis structure of water h2o? Web 6 steps to draw the lewis structure of h2o. A common error it to put two oxygen atoms and one hydrogen making ho 2. These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other. In order to find the total valence electrons in h2o molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Make sure you put the correct atom at the center of the water (h 2 o) molecule. In this article, we will delve into the details of the h2o lewis structure, including how to draw it, its properties, and faqs. Find the total valence electrons in h2o molecule. Web steps of drawing h2o lewis structure. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure.
H2o water molecule model chemical formula Vector Image
Make sure you put the correct atom at the center of the water (h 2 o) molecule. See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. In order to find the total valence electrons in h2o molecule, first of all you should know the valence electrons present in hydrogen atom as.
Draw Step By Step The Lewis Structure For Water (H2O)
Make sure you put the correct atom at the center of the water (h 2 o) molecule. Remember that hydrogen only needs two electrons to have a full outer shell. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: Water has tetrahedral shape, or to be more precise, bent shape. Determine the total.
Lewis Dot Diagram For H2o Free Diagram For Student
Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. It is eight to form a single h2o molecule. See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. For the h2o structure use the periodic table to find the total number of valence electrons for.
H2O Water molecule model and chemical formula Digital Art by Peter
840 views 4 years ago lewis structure (chemistry) how to draw lewis structure for h2o water lewis structure: Drawing the lewis dot structure for h2o and answer the questions below. In order to find the total valence electrons in h2o molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. See.
H2O Chemical Medical Formula for Water Molecula in Blue Color. Simple
Web i quickly take you through how to draw the lewis structure of water, h2o. Look for how many electrons are needed: (valence electrons are the electrons that are present in the outermost orbit of any. Let's do the lewis structure for h2o2: Since h 2 o has two hydrogen atoms and one oxygen.
H2O Molecule 3d illustration Stock Photo 67864280 Alamy
Because the water molecule has four electron domains (the two hydrogen. A simple notation used to represent valence electrons in an atom is called lewis. I also go over hybridization, shape and bond angle. Calculate the total number of valence electrons. It is four for one water (h2o) molecule according to the octet rule.
H2o water molecule Royalty Free Vector Image VectorStock
• how to draw lewis. Look at the questions before watching the video. Hydrogen peroxide, also called dihydrogen dioxide. O has 6 valence electrons, and each h has one. Web watch the video of dr.
Water Lewis Structure How to Draw the Lewis Structure for Water YouTube
For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. On this picture you can see tetrahedral shapes of water, ammonia and methane. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Drawing the lewis structure for water. 840 views 4 years ago lewis.
Chemistry model of molecule water H2O scientific elements. Integrated
Web 6 steps to draw the lewis structure of h2o. Water has tetrahedral shape, or to be more precise, bent shape. Hence, hydrogen has one valence electron and oxygen has six valence electrons. For h₂o, o must be the central atom. Make sure you have two hydrogens and one oxygen in h 2 o!
H2O Lewis Structure, Molecular Geometry, and Hybridization
Here, the given molecule is h2o (water). Web steps of drawing h2o lewis structure. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: (valence electrons are the electrons that are present in the outermost orbit of any. Water has tetrahedral shape, or to be more precise, bent shape.
Look At The Questions Before Watching The Video.
Web lewis structure of water molecule contains two single bonds. Web steps of drawing h2o lewis structure. First, determine the total number of valence electrons. Drawing the lewis dot structure for h2o and answer the questions below.
How To Draw The Dot Structure For H2O | Chemical Bonding | Success In Chemistry.
Remember that hydrogen only needs two electrons to have a full outer shell. Web understanding the lewis structure of h2o is crucial to understanding the properties and behavior of water, which is an essential substance for life. For h₂o, o must be the central atom. Web drawing the lewis structure of h2o helps us visualize the arrangement of atoms and valence electrons in the molecule.
In This Video We Look At The Electron Geometry For Water (H2O).
To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. Since h 2 o has two hydrogen atoms and one oxygen. But it is better to say that methane has tetrahedral shape,. Water has tetrahedral shape, or to be more precise, bent shape.
In Order To Find The Total Valence Electrons In H2O Molecule, First Of All You Should Know The Valence Electrons Present In Hydrogen Atom As Well As Oxygen Atom.
You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. A common error it to put two oxygen atoms and one hydrogen making ho 2. Look for the total valence electrons: For the h2o structure use the periodic table to find the total number of valence.