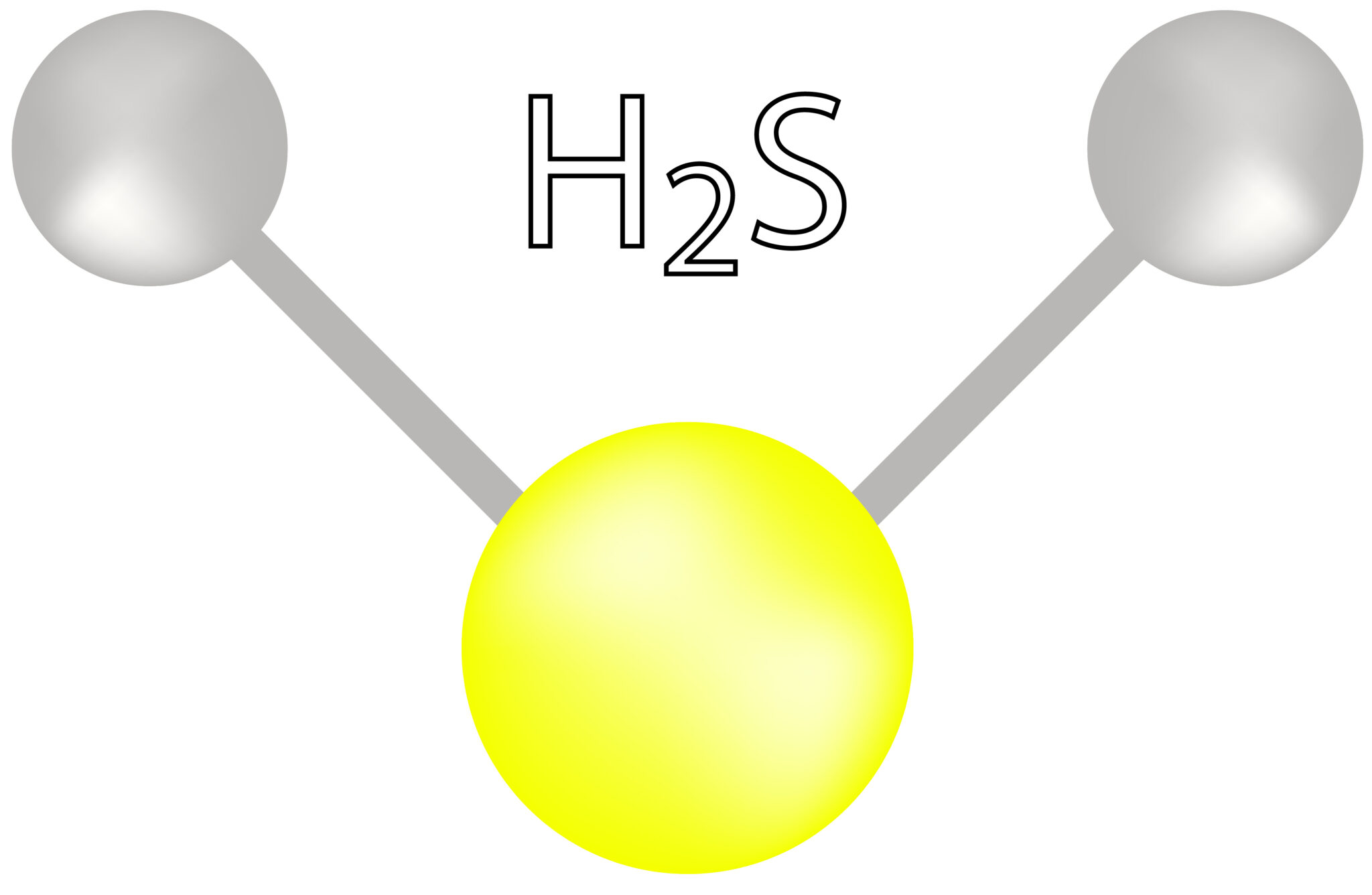
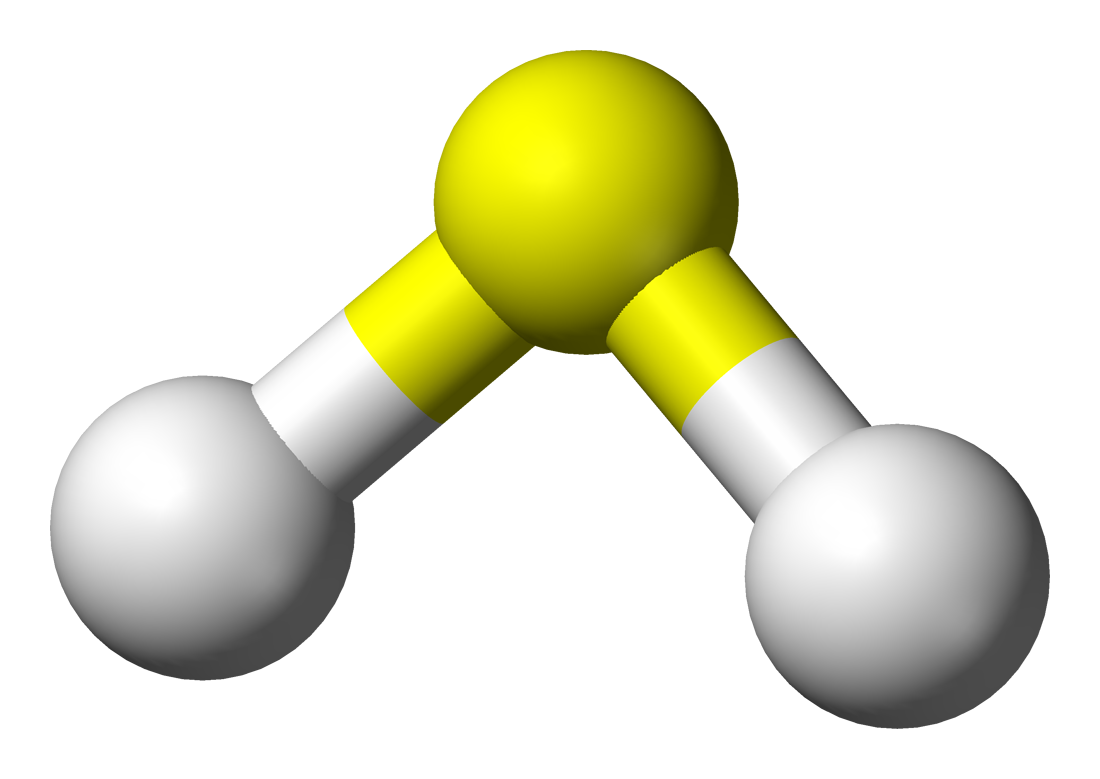
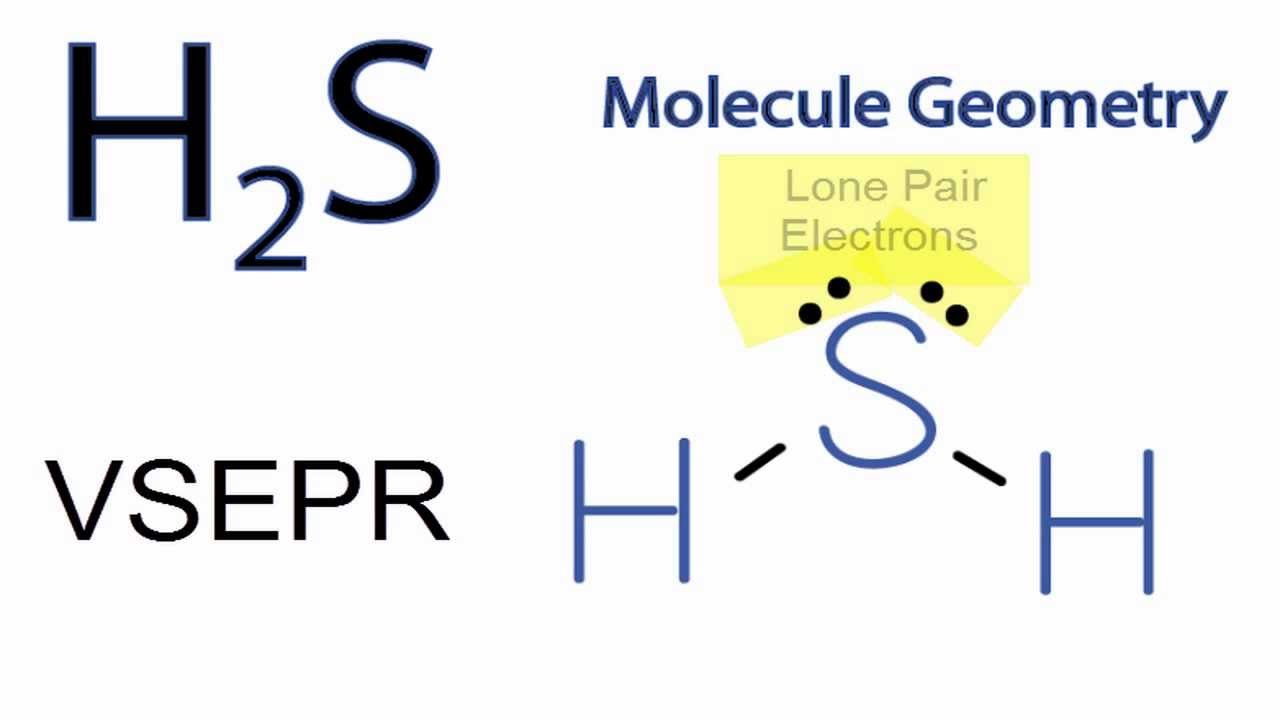
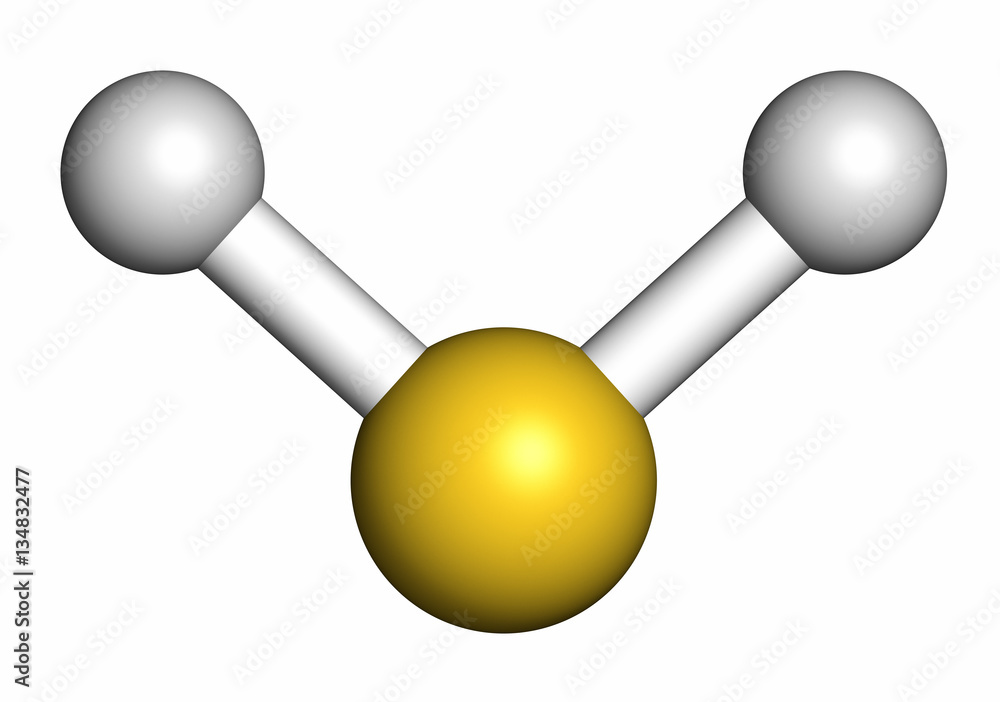
H2S 3D Drawing
H2S 3D Drawing - In accordance with 40 cfr part 23, this regulation shall be considered issued for purposes of judicial review at 1 p.m. Sulfur needs eight electrons to fulfill the requirements for octet rule. 166k views 10 years ago. The 3d structure may be viewed using java or javascript. The h2s lewis structure is similar to the structure for water h2o since sulfur (s) and (o) are. Ends in 0 d 0 hrs 0 mins 0 secs. Webgl appears to be disabled. Pick one of the bond types (single, double, triple, up, down) and add or modify bonds. Note that actual 3d physical models and web activities can be very helpful in solidifying your ideas about structure. Generally the atom with the highest bonding sites.
This final rule is effective on july 8, 2024. Each provides specific kinds of information about the molecule. To predict whether a molecule has a dipole moment. How does molecule shape change with different numbers of bonds and electron pairs? Find total number of electrons of the valance shells of hydrogen atoms and sulfur atom. Regarding its molecular symmetry, it belongs to the c 2v point group. Webgl appears to be disabled. In such cases, they are mentioned with those respective steps. Co 2 (carbon dioxide) 7. Those steps are explained in detail in this tutorial.
Fill up the octet of the atoms with the remaining electrons. Web may 22, 2023 by jay rana. To predict whether a molecule has a dipole moment. Create a chain of carbon atoms. Eastern time on may 23, 2024.under section 509(b)(1) of the clean water act. Hydrogen has one, and sulfur has six valence electrons. Web the lewis structure of hydrogen sulfide is easy to draw and understand. In addition, it has a dipole moment of 0.97 debye. Each provides specific kinds of information about the molecule. Find total number of electrons of the valance shells of hydrogen atoms and sulfur atom.
Library of Structures Chemistry, Structures
The h2s lewis structure is similar to the structure for water h2o since sulfur (s) and (o) are. Controls () how useful was this page? Co 2 (carbon dioxide) 7. Then, compare the model to real molecules! Web you have probably already seen some of these different structures, and we will consider a number of them below.
Understanding Hydrogen Sulfide (H2S) Aulick Chemical Solutions
166k views 10 years ago. Web drawing lewis structures for molecules with one central atom: The 3d structure may be viewed using java or javascript. In this compound, both the hydrogen atoms require one electron to make the covalent bond with sulfur. Generally the atom with the highest bonding sites.
FileHydrogensulfide3Dballs.png
In accordance with 40 cfr part 23, this regulation shall be considered issued for purposes of judicial review at 1 p.m. Eastern time on may 23, 2024.under section 509(b)(1) of the clean water act. Web drawing organic compounds in 3d. To predict whether a molecule has a dipole moment. Hydrogen (h) is located in group 1, and sulfur (s) is.
H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity
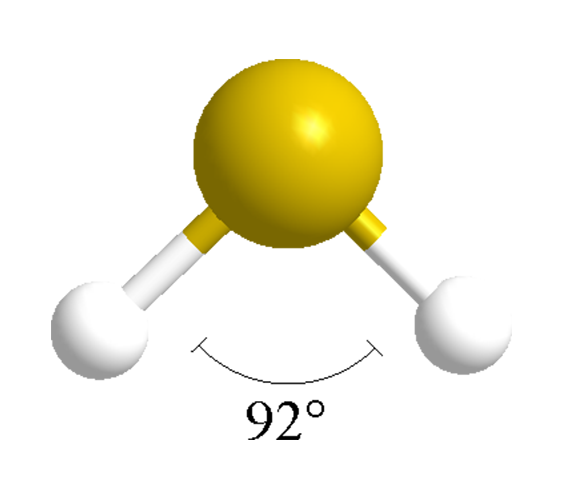
Web molecular geometry of hydrogen sulfide (h. This final rule is effective on july 8, 2024. In addition, it has a dipole moment of 0.97 debye. Hydrogen has one, and sulfur has six valence electrons. A quick explanation of the molecular geometry of h2s including a description of the h2s bond angles (note:
H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Web molecular geometry of hydrogen sulfide (h. Web drawing organic compounds in 3d. To use the vsepr model to predict molecular geometries. Web interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university courses and advanced school chemistry hosted by university of liverpool. Eastern time on may 23, 2024.under section 509(b)(1) of the.
Hydrogen sulfide (H2S) molecule, 3D rendering. Stock Illustration
The h2s lewis structure is similar to the structure for water h2o since sulfur (s) and (o) are. Total of 8 valence electrons. The total number of valence electrons in hydrogen sulfide is 8. Draw a skeletal structure with single bonds only. Then, compare the model to real molecules!
H2s molecular geometry singleatila
There are multiple ways to represent molecules in 3d: Note that actual 3d physical models and web activities can be very helpful in solidifying your ideas about structure. Web there are several steps to draw the lewis structure of h 2 s. Co 2 (carbon dioxide) 7. In addition, it has a dipole moment of 0.97 debye.
H2S (Hydrogen sulfide) Molecular Geometry, Bond Angles YouTube
Sulfur needs eight electrons to fulfill the requirements for octet rule. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Webgl appears to be disabled. Web the lewis structure of hydrogen sulfide is easy to draw and understand. Web there are several steps to draw the lewis structure of h 2.
Hydrogen Sulfide Lewis Structure
Co 2 (carbon dioxide) 7. The h2s lewis structure is similar to the structure for water h2o since sulfur (s) and (o) are. Because h 2 s molecule is a simple molecule, some of these steps are not used. Learn how molecule shapes change with different bonds and electron pairs. Plus sulfur is in group 6 or 16 on the.
H2S Molecular Geometry Science Education and Tutorials
Find total number of electrons of the valance shells of hydrogen atoms and sulfur atom. Eastern time on may 23, 2024.under section 509(b)(1) of the clean water act. Regarding its molecular symmetry, it belongs to the c 2v point group. How does molecule shape change with different numbers of bonds and electron pairs? In this compound, both the hydrogen atoms.
Ends In 0 D 0 Hrs 0 Mins 0 Secs.
Create a chain of carbon atoms. Using formal charges to determine how many bonds to make, a different perspective. Plus sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Note that actual 3d physical models and web activities can be very helpful in solidifying your ideas about structure.
Drawing Lewis Structures For Bf3, Pf3 And Brf3;
Total of 8 valence electrons. 40k views 2 years ago lewis structures. This final rule is effective on july 8, 2024. How does molecule shape change with different numbers of bonds and electron pairs?
The 3D Structure May Be Viewed Using Java Or Javascript.
Breaking the octet rule ; Web build and explore molecules in 3d with this interactive simulation. Web you have probably already seen some of these different structures, and we will consider a number of them below. Eastern time on may 23, 2024.under section 509(b)(1) of the clean water act.
Find Total Number Of Electrons Of The Valance Shells Of Hydrogen Atoms And Sulfur Atom.
Springsale and get 50% off 3d models 🌸. Regarding its molecular symmetry, it belongs to the c 2v point group. The total number of valence electrons in hydrogen sulfide is 8. In addition, it has a dipole moment of 0.97 debye.