How Do Nonmetals Form Negative Ions
How Do Nonmetals Form Negative Ions - Metals tend to form cations, while nonmetals tend to form anions. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. These are electronegative elements with high ionization. Negatively charged ions are called. By bonding with other negative ions. How do nonmetals form negative ions? Thus, the electron affinity will be. Negatively charged ions are called. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. By losing one or more electrons.
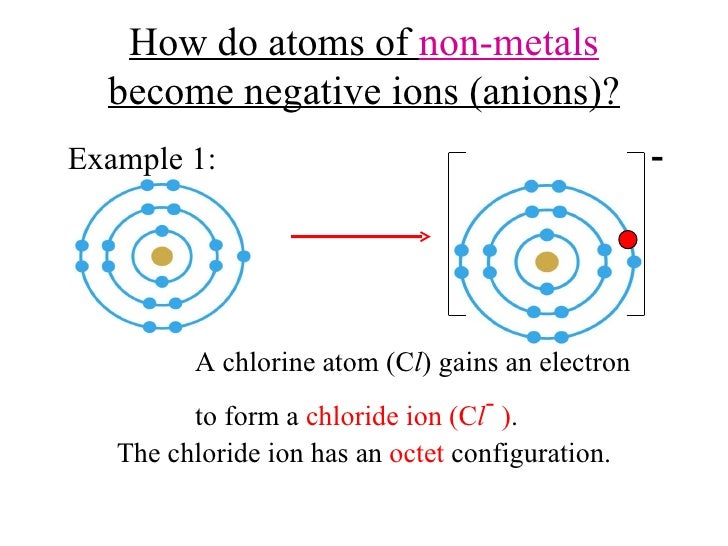
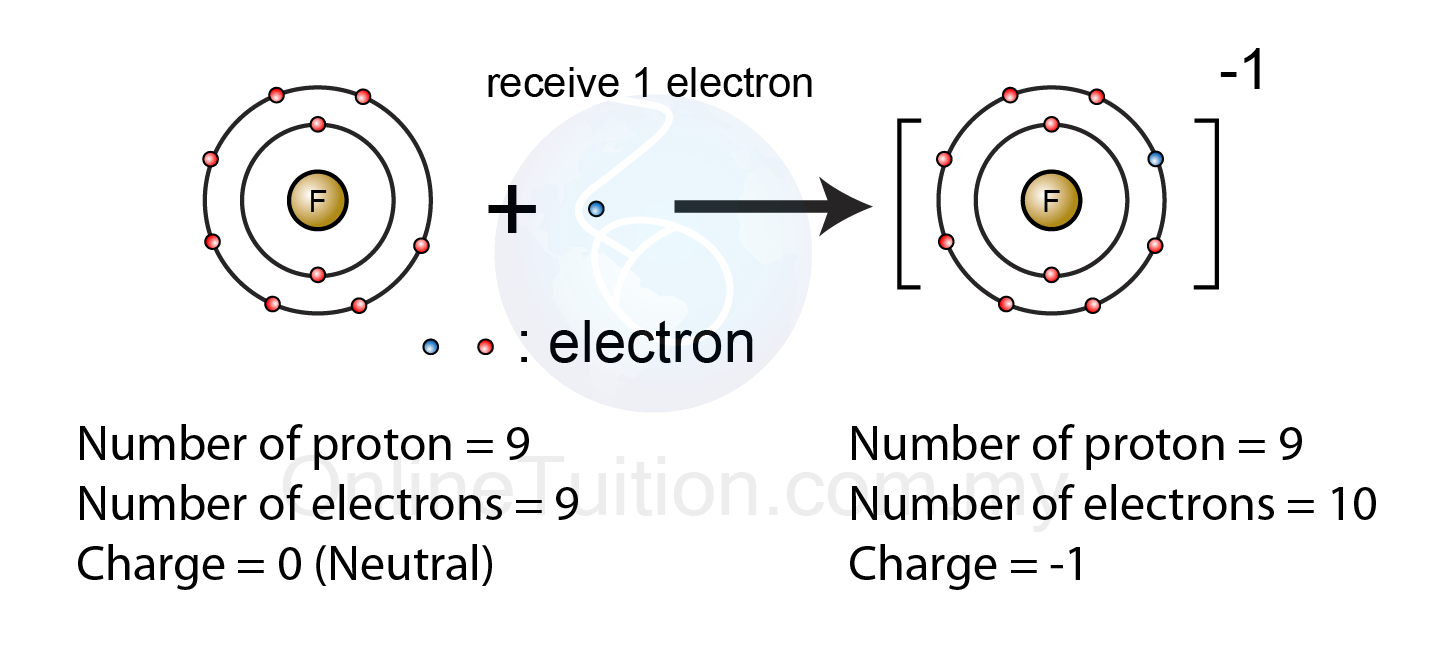
Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); The ions formed are negative, because they have more electrons than protons the ions have the. Web they gain electrons to become negative or an ion. By losing one or more. By losing one or more electrons. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. To obtain a full outer shell: How do nonmetals form ions? Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.
Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. To obtain a full outer shell: Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Negatively charged ions are called. Negatively charged ions are called. Second, most atoms form ions of a single. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Web they gain electrons to become negative or an ion. How do nonmetals form negative ions? Web thus, nonmetals tend to form negative ions.
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
By losing one or more electrons. By bonding with other negative ions. Ions can be either monatomic (containing only. These are electronegative elements with high ionization. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table.
table of elements chart
How do nonmetals form negative ions? Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Ions can be either monatomic (containing only. The ions formed are negative, because they have more electrons than protons the ions have the. How do nonmetals form ions?
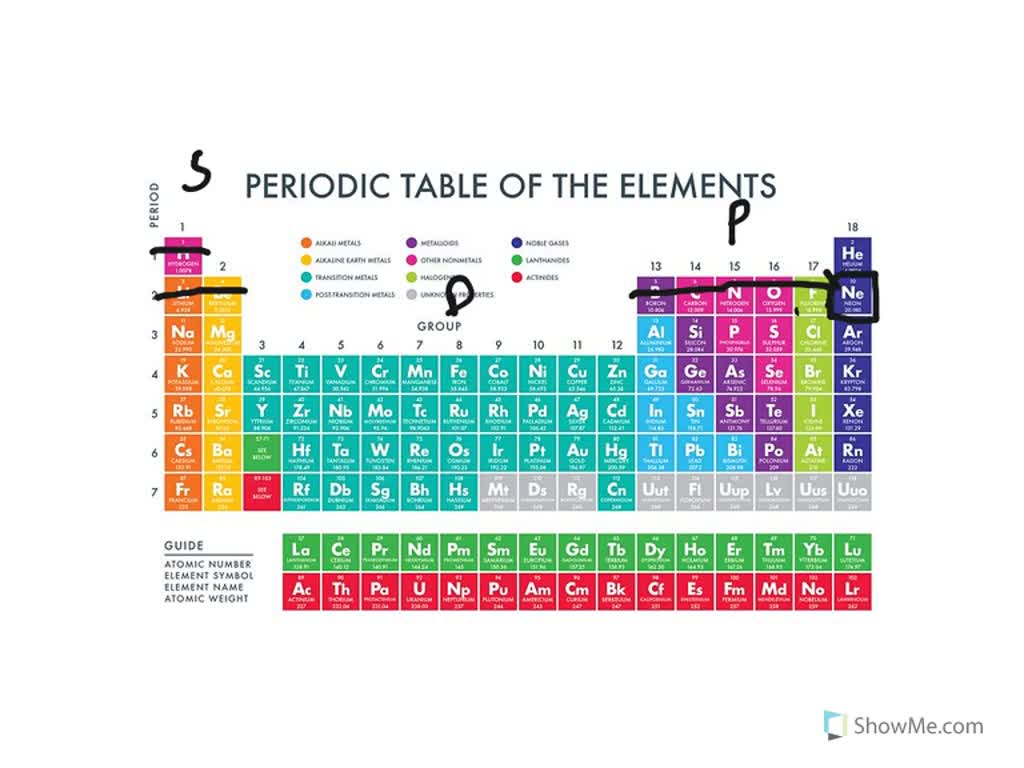
The Parts of the Periodic Table
Second, most atoms form ions of a single. Web thus, nonmetals tend to form negative ions. Metals tend to form cations, while nonmetals tend to form anions. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Web when these atoms gain electrons, they acquire a negative charge because they now.
Nonmetals and anion formation YouTube
See answer (1) best answer. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Ions can be either monatomic (containing only. Metals tend to form cations, while nonmetals tend to form anions. Positively charged ions are called cations, and negatively charge ions are called anions.
Hydrogen Valence Electrons In Hydrogen
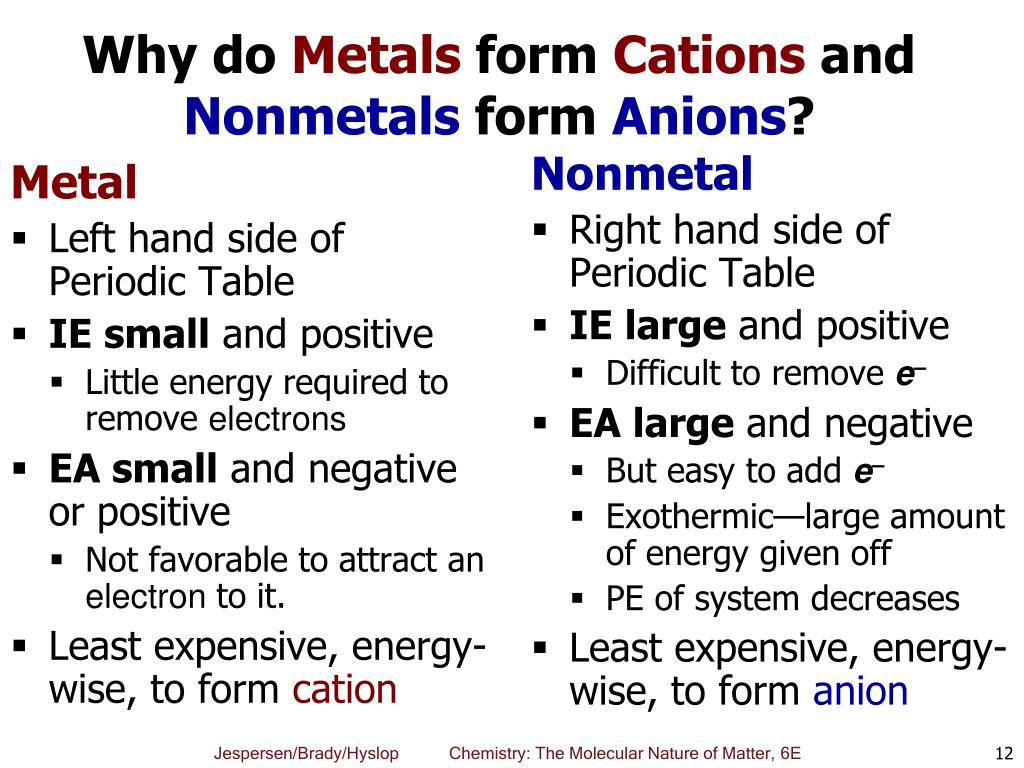
Web this is actually one of the chemical properties of metals and nonmetals: Negatively charged ions are called. By losing one or more electrons. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. Web when these atoms gain electrons, they acquire a negative charge because they.
Chem matters ch6_ionic_bond
By losing one or more. Ions can be either monatomic (containing only. Web ions form when atoms lose or gain electrons. By bonding with other negative ions. Web this is actually one of the chemical properties of metals and nonmetals:
Electron Affinity of The Elements
How do nonmetals form ions? Web ions form when atoms lose or gain electrons. By losing one or more. Negatively charged ions are called. Web thus, nonmetals tend to form negative ions.
Formation of Negative Ions SPM Chemistry
Fx−, clx−, ix−, sx2− f x −, c l x −, i x −, s x 2 − multi. Web they gain electrons to become negative or an ion. How do nonmetals form ions? Web thus, nonmetals tend to form negative ions. Metals tend to form cations, while nonmetals tend to form anions.
SOLVEDNonmetals form negative ions by (losing/gaining) enough
Positively charged ions are called cations, and negatively charge ions are called anions. Ions can be either monatomic (containing only. By losing one or more electrons. How do nonmetals form ions? How do nonmetals form negative ions?
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Positively charged ions are called cations, and negatively charge ions are called anions. Metals tend to form cations, while nonmetals tend to form anions. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web thus, nonmetals tend to form negative ions. Negatively charged ions are called.
How Do Nonmetals Form Negative Ions?
The ions formed are negative, because they have more electrons than protons the ions have the. Positively charged ions are called cations, and negatively charge ions are called anions. Web they gain electrons to become negative or an ion. Thus, the electron affinity will be.
Negatively Charged Ions Are Called.
Web this is actually one of the chemical properties of metals and nonmetals: Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Fx−, clx−, ix−, sx2− f x −, c l x −, i x −, s x 2 − multi. How do nonmetals form ions?
See Answer (1) Best Answer.
To obtain a full outer shell: Negatively charged ions are called. Web thus, nonmetals tend to form negative ions. These are electronegative elements with high ionization.
Web When Nonmetals Gain Electrons, The Energy Change Is Usually Negative Because They Give Off Energy To Form An Anion (Exothermic Process);
By losing one or more electrons. Web when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Metals tend to form cations, while nonmetals tend to form anions. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table.