How Many Bonds Can Helium Form
How Many Bonds Can Helium Form - Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Two hydrogen atoms can form covalent bond because they only have two electrons in. How many hydrogen bonds can ch,o make to water? The energy required for breaking the bonds is as follows: Web helium (he), neon (ne), and argon (ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. Web after hydrogen, helium is the second most abundant element in the universe. These h − h molecules are themselves. See answer (1) best answer. Web helium already has 2, so it will typically make zero bonds. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms.
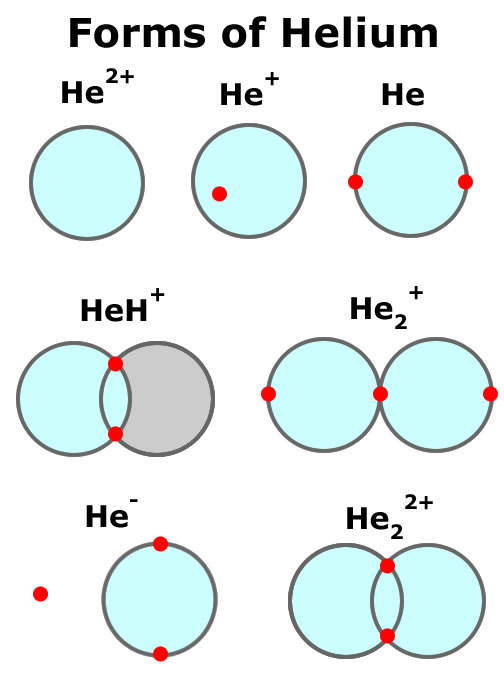
How many hydrogen bonds can ch,o make to water? Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along. Web helium already has 2, so it will typically make zero bonds. There is only one shell of electrons, the valence shell of two electrons. Upvote • 0 downvote add comment report still looking for help? Since ch2o has two hydrogen atoms hence it can form two hydrogen bond with oxygen. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted. How many bonds can helium form, helium: It is present in all stars. Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot?
The energy required for breaking the bonds is as follows: Web helium (he), neon (ne), and argon (ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. How many bonds can helium form, helium: Web after hydrogen, helium is the second most abundant element in the universe. Web the two chlorine atoms are said to be joined by a covalent bond. In order to bond it to another element, it would. Web of all the elements, helium is the least reactive, so it does not form compounds under ordinary conditions. Since ch2o has two hydrogen atoms hence it can form two hydrogen bond with oxygen. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted. It is a noble gas and is ths relatively.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web the two chlorine atoms are said to be joined by a covalent bond. Web helium already has 2, so it will typically make zero bonds. Get the right answer, fast. These h − h molecules are themselves. Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot?
Helium Form Periodic Table of Elements Stock Vector Illustration of
Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along. The energy required for breaking the bonds is as follows: Upvote • 0 downvote add comment report still looking for help? It is a noble gas and is ths relatively. Web helium already has 2, so it will typically make zero bonds.
ASSTUDYPEACH Covalent Bonds Sharing Is Caring!
This makes them highly stable as single atoms. There is only one shell of electrons, the valence shell of two electrons. Get the right answer, fast. How many bonds can helium form, helium: Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along.
Vast new reserves of helium discovered Cosmos Chemistry education
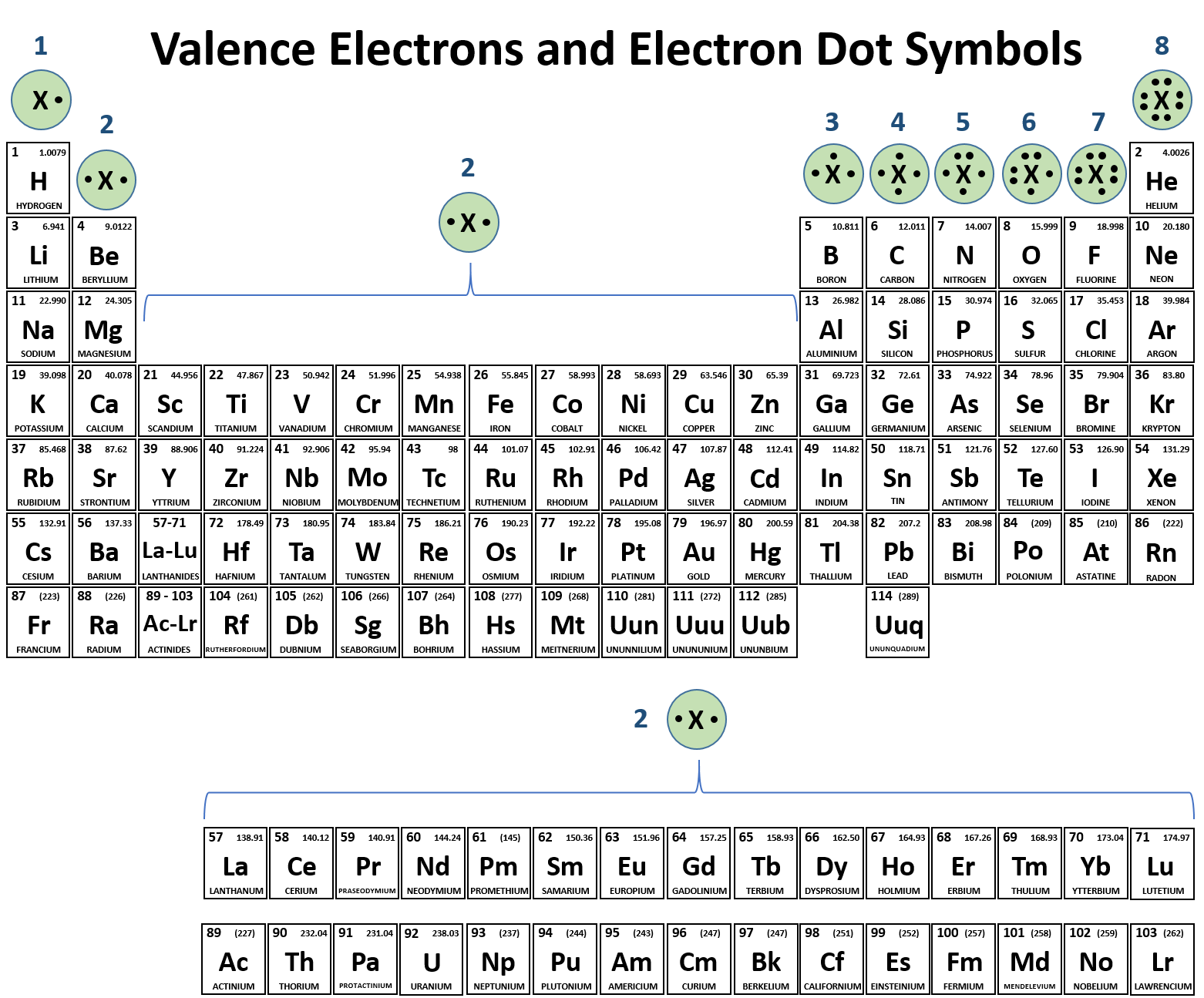
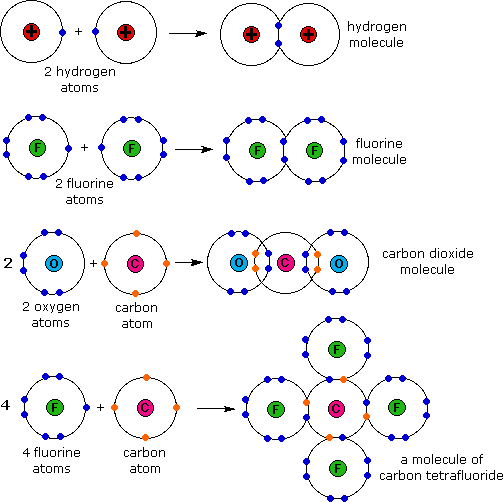
Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. We can see this in h2 (hydrogen gas), h2o (water), and hcl (hydrochloric acid). Get the right answer, fast. It is a noble gas and is ths relatively. There is only one shell of electrons, the valence shell of two electrons.
__TOP__ How Many Covalent Bonds Can Chlorine Form
How many bonds can helium form, helium: This makes them highly stable as single atoms. Web the two chlorine atoms are said to be joined by a covalent bond. These h − h molecules are themselves. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted.
How Many Valence Electrons Does Helium (He) Have? [Valency of He]
These h − h molecules are themselves. Web after hydrogen, helium is the second most abundant element in the universe. Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot? There is a quick way to work out how many covalent bonds an element will. Web of all the elements, helium is the least reactive, so.
How to Predict number of bonds each element forms ChemSimplified
This makes them highly stable as single atoms. It is a noble gas and is ths relatively. Two hydrogen atoms can form covalent bond because they only have two electrons in. Web the two chlorine atoms are said to be joined by a covalent bond. Since ch2o has two hydrogen atoms hence it can form two hydrogen bond with oxygen.
representation molecule (Lewis, valence)
The energy required for breaking the bonds is as follows: Get the right answer, fast. There is only one shell of electrons, the valence shell of two electrons. How many hydrogen bonds can ch,o make to water? Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot?
Up, up and away Chemists say 'yes,' helium can form compounds
In each case hydrogen will share with the other. There is a quick way to work out how many covalent bonds an element will. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted. Web of all the elements, helium is the least reactive, so it does not form compounds under ordinary.
Hydrogen energy levels are not quite exact.
Get the right answer, fast. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted. Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot? Web a helium atom is an atom of the chemical element helium. How many hydrogen bonds can ch,o make to water?
The Energy Required For Breaking The Bonds Is As Follows:
How many bonds can helium form, helium: See answer (1) best answer. Web hydrogen can only form one covalent bond. Web helium already has 2, so it will typically make zero bonds.
It Is Present In All Stars.
It is a noble gas and is ths relatively. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along. How many hydrogen bonds can ch,o make to water? Get the right answer, fast.
Web After Hydrogen, Helium Is The Second Most Abundant Element In The Universe.
The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted. Helium atoms have two electrons and two protons. Web helium (he), neon (ne), and argon (ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. Web each h can form only a single covalent bond, leading to the formation of h − h molecules, which are often also written as h 2 molecules.
Since Ch2O Has Two Hydrogen Atoms Hence It Can Form Two Hydrogen Bond With Oxygen.
We can see this in h2 (hydrogen gas), h2o (water), and hcl (hydrochloric acid). Upvote • 0 downvote add comment report still looking for help? In order to bond it to another element, it would. This makes them highly stable as single atoms.




![How Many Valence Electrons Does Helium (He) Have? [Valency of He]](https://1.bp.blogspot.com/-YNupZqYfI0Y/YACG_7--PTI/AAAAAAAADO0/um0r48XLVG8WVR-Xg5krtG2wU-eD7H6JgCLcBGAsYHQ/s832/Screenshot%2B%252857%2529.png)