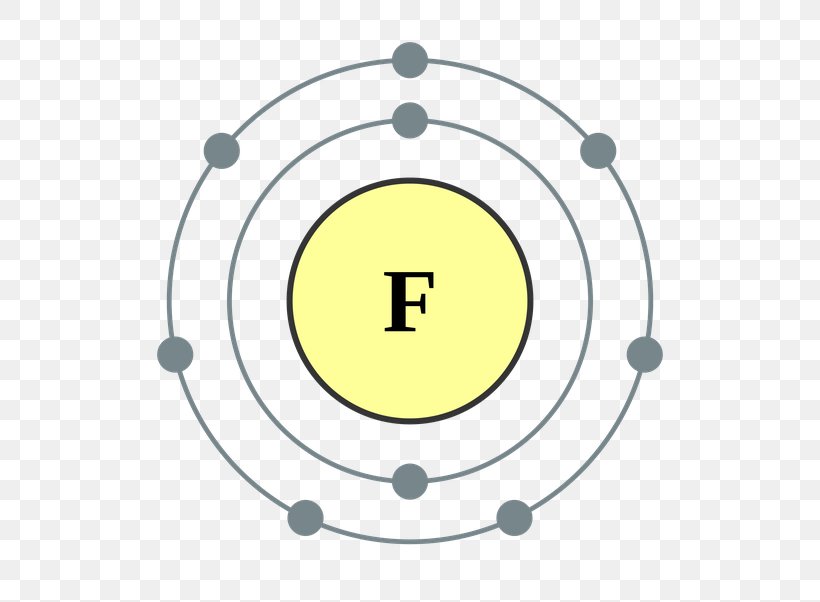
How Many Bonds Does Fluorine Form
How Many Bonds Does Fluorine Form - With these electron configurations, none of these atoms will have any formal charge. Web describe the important exceptions to the octet rule. There are six fluorine atoms that form bonds. Web continuing on across the periodic table we see that fluorine is the next element after oxygen. One bond it has 9 electrons, 2 core and 7 valence. In higher level chemistry, it may be possible (like in the. Web how many hydrogen bonds can fluorine make? As a stable electron configuration requires 8 electrons total, fluorine must form 1 bond i.e…. Rather than forming 7 bonds, fluorine only forms a. Based on the electron configuration of fluorine, how many bonds does fluorine form even if it does not undergo hybridization?
What type of bonds does fluorine and calcium make?. Web for most purposes, i.e. One bond it has 9 electrons, 2 core and 7 valence. Web chemistry questions and answers. Web how many hydrogen bonds can fluorine make? Which atoms do not usually form bonds? Web fluorine (and all halogens) tends to form one bond and have 3 lone pairs. The atom would be energetically stable if it achieved an 8 electron count. In higher level chemistry, it may be possible (like in the. Web 1 bond a fluorine atom (by itself) has 7 valence electrons.
Rather than forming 7 bonds, fluorine. The atom would be energetically stable if it achieved an 8 electron count. Rather than forming 7 bonds, fluorine only forms a. So it form 1 covalent bo. Two core and seven valence. In the case of boron in bf 3, three bonds is the maximum possible because. In higher level chemistry, it may be possible (like in the. Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. There are six fluorine atoms that form bonds. Web fluorine (and all halogens) tends to form one bond and have 3 lone pairs.
Covalent Bonding (Biology) — Definition & Role Expii
So it form 1 covalent bo. Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. It has 9 electrons, 2 core and 7 valence. Web describe the important exceptions to the octet rule. The polar nature of the bond means that there is a large.
How Many Bonds Can Magnesium Form TheFitnessManual
So it form 1 covalent bo. Web for most purposes, i.e. Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Web how many hydrogen bonds can fluorine make? Writing lewis structures and such things, fluorine always forms single bonds.
氟(一种非金属化学元素)_百度百科
One bond it has 9 electrons, 2 core and 7 valence. Web how many hydrogen bonds can fluorine make? Rather than forming 7 bonds, fluorine. In higher level chemistry, it may be possible (like in the. Web 1 bond a fluorine atom (by itself) has 7 valence electrons.
How Many Valence Electrons Does Fluorine Have doodledesignit
Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. Web continuing on across the periodic table we see that fluorine is the next element after oxygen. Which atoms do not usually form bonds? In higher level chemistry, it may be possible (like in the. So it form 1 covalent.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Atomic fluorine has 7 valence electrons; Web a single covalent bond. Which atoms do not usually form bonds? One bond it has 9 electrons, 2 core and 7 valence. It has 9 electrons, 2 core and 7 valence.
How Many Valence Electrons Does Fluorine Have?
Rather than forming 7 bonds, fluorine. What type of bonds does fluorine and calcium make?. Web how many hydrogen bonds can fluorine make? Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine.
Periodic Table Fluorine Valence Electrons Periodic Table Timeline
Web continuing on across the periodic table we see that fluorine is the next element after oxygen. So it form 1 covalent bo. One bond it has 9 electrons, 2 core and 7 valence. In the case of boron in bf 3, three bonds is the maximum possible because. Which atoms do not usually form bonds?
Fluorine (F) Properties & Uses StudiousGuy
So it form 1 covalent bo. Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. One bond it has 9 electrons, 2 core and 7 valence. Web chemistry questions and answers. Rather than forming 7 bonds, fluorine.
How Many Valence Electrons Does Fluorine Have doodledesignit
Which atoms do not usually form bonds? In higher level chemistry, it may be possible (like in the. Web how many hydrogen bonds can fluorine make? Web describe the important exceptions to the octet rule. Web for most purposes, i.e.
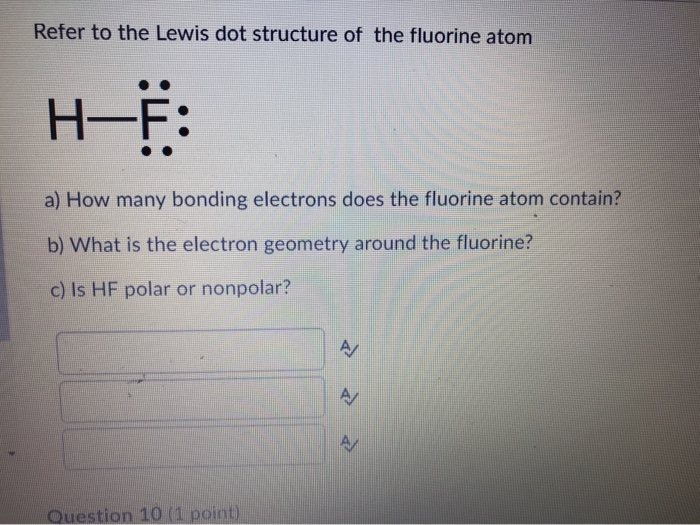
Solved Refer to the Lewis dot structure of the fluorine atom
The atom would be energetically stable if it achieved an 8 electron count. Rather than forming 7 bonds, fluorine only forms a. The polar nature of the bond means that there is a large. Web chemistry questions and answers. As a stable electron configuration requires 8 electrons total, fluorine must form 1 bond i.e….
Web The High Electronegativity Of Fluorine Means That It Forms A Single Electron Pair Bond Polar Bond With A High Ionic Character.
One bond it has 9 electrons, 2 core and 7 valence. In higher level chemistry, it may be possible (like in the. Web fluorine (and all halogens) tends to form one bond and have 3 lone pairs. With these electron configurations, none of these atoms will have any formal charge.
Web For Most Purposes, I.e.
Web a single covalent bond. Atomic fluorine has 7 valence electrons; Writing lewis structures and such things, fluorine always forms single bonds. Web the elements that receive electrons and form bonds are called anion.
Two Core And Seven Valence.
It has 9 electrons, 2 core and 7 valence. Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. What type of bonds does fluorine and calcium make?. Which atoms do not usually form bonds?
There Are Six Fluorine Atoms That Form Bonds.
Web continuing on across the periodic table we see that fluorine is the next element after oxygen. Web 1 bond a fluorine atom (by itself) has 7 valence electrons. In the case of boron in bf 3, three bonds is the maximum possible because. As a stable electron configuration requires 8 electrons total, fluorine must form 1 bond i.e….