How Many Covalent Bonds Can Sulfur Form
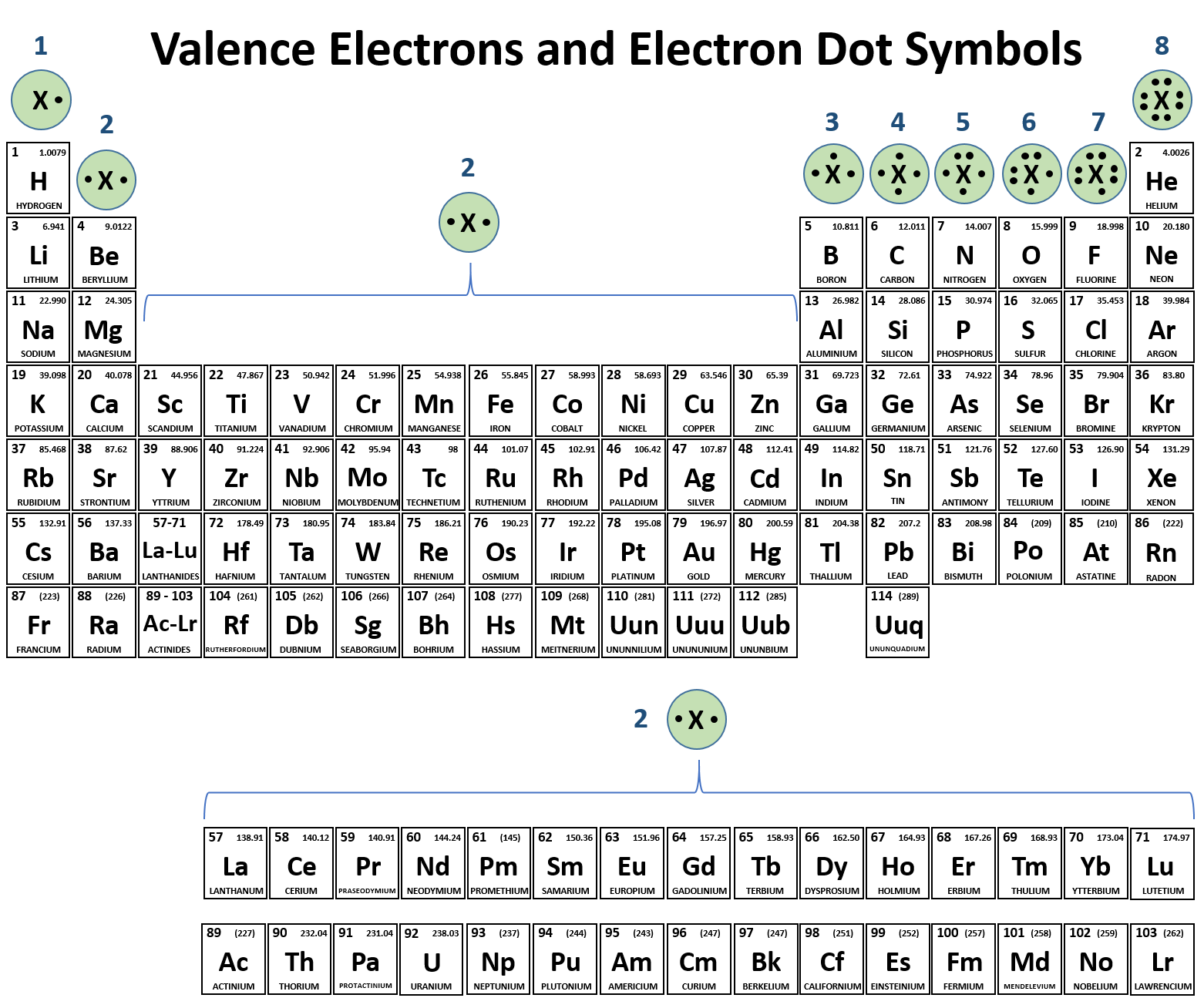
How Many Covalent Bonds Can Sulfur Form - Web each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the phosphorus's unpaired electrons to. Web the nucleus of a sulfur atom wil have 16 neutron and two hydrogen atoms can form covalent bond with sulfur. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. Web most of the time a sulfur atom can form two bonds. Covalent bonds are formed when two non metallic elements combine by sharing their electrons. Web covalent radius half of the distance between two atoms within a single covalent bond. Its valency is only −2 − 2, it only needs two electrons, yet here it's getting 6 6. It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. Web why does sulfur form so many covalent bonds.
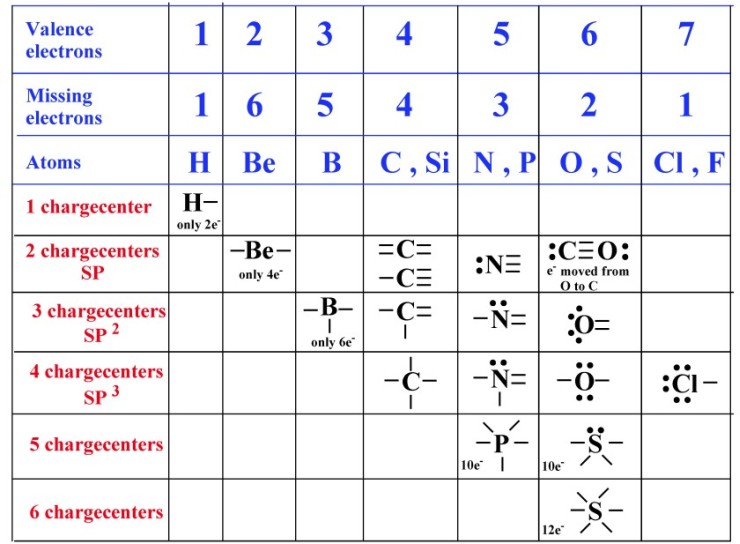
Web can sulfur make 6 bonds? It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. In elemnatl allotropes of sulfur which are covalent bonded, many are cyclic compounds the. Web how many covalent bonds can sulfur form? Web how many bonds should be attached to sulfur? There is a quick way to work out how many covalent bonds an element. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. In order to obey the octet rule, it needs. Web each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the phosphorus's unpaired electrons to. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the.
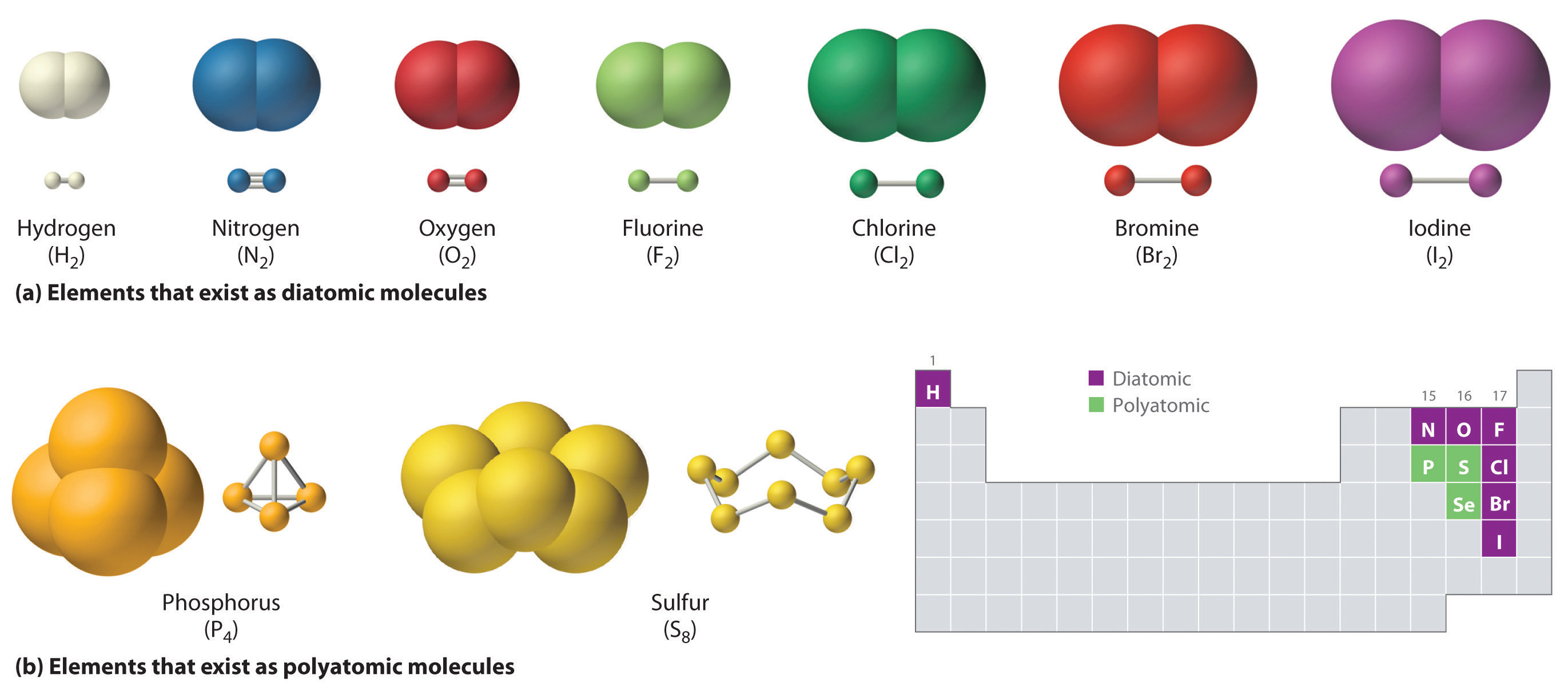
Web sulfur can form two covalent bonds as in h2s, and can form 6 as in so3. The key to this problem is that electrons in. Web how many covalent bonds can sulfur form? Web up to 6% cash back how many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule? There is a quick way to work out how many covalent bonds an element. Web the nucleus of a sulfur atom wil have 16 neutron and two hydrogen atoms can form covalent bond with sulfur. It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. Web covalent radius half of the distance between two atoms within a single covalent bond. Web most of the time a sulfur atom can form two bonds. In order to obey the octet rule, it needs.
How Good is the Sulfur Atom as Hydrogen Bond Acceptor? Chemistry
The key to this problem is that electrons in. In general, sulfur can form covalent bonds by sharing its valence electrons with other atoms. In order to obey the octet rule, it needs. Web the nucleus of a sulfur atom wil have 16 neutron and two hydrogen atoms can form covalent bond with sulfur. Web how many bonds should be.
Covalent Bonds Biology for NonMajors I
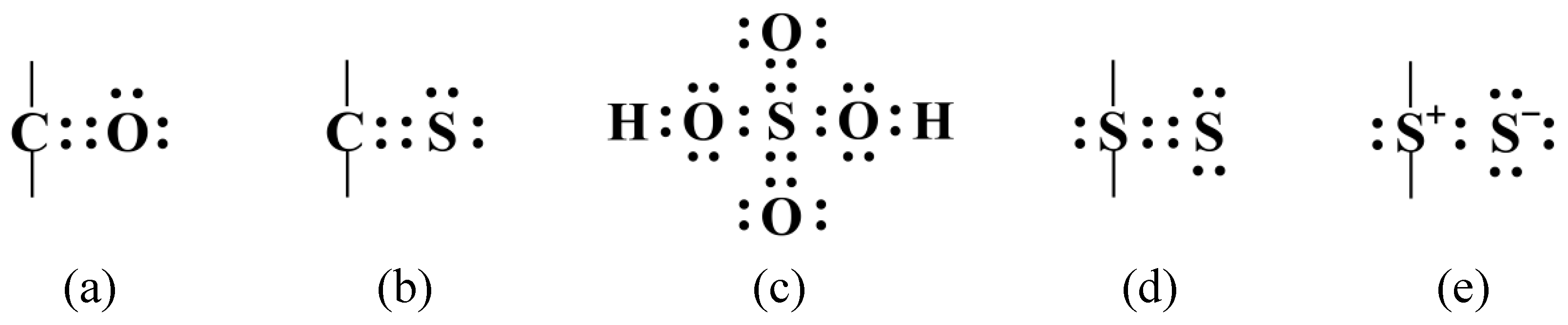
Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. Web covalent radius half of the distance between two atoms within a single covalent bond. Will form either one, two, three or four covalent bonds with other atoms. Sulfur is a nonmetal in.
how many bonds does sulfur form
The key to this problem is that electrons in. Web the maximum number of covalent bonds sulfur can form is two. Web up to 6% cash back how many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule? Web the total number of electrons around each individual atom consists of six.
how many bonds does sulfur form
Sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons. The key to this problem is that electrons in. Web most of the time a sulfur atom can form two bonds. The same thing happens with phosphate. Covalent bonds are formed when two non metallic elements combine by sharing their electrons.
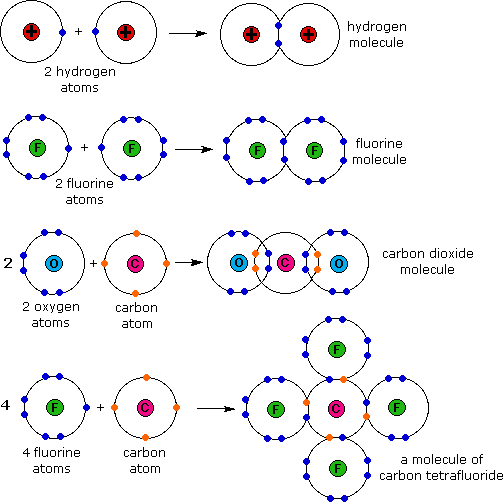
What's the difference between a formula unit and a molecule? Socratic
Web sulfur can form two covalent bonds as in h2s, and can form 6 as in so3. In order to obey the octet rule, it needs. Web covalent radius half of the distance between two atoms within a single covalent bond. A covalent bond is an. Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web covalent radius half of the distance between two atoms within a single covalent bond. Web how many bonds should be attached to sulfur? Its valency is only −2 − 2, it only needs two electrons, yet here it's getting 6 6. In elemnatl allotropes of sulfur which are covalent bonded, many are cyclic compounds the. Web most of the.
polarity Definition & Examples Britannica
In elemnatl allotropes of sulfur which are covalent bonded, many are cyclic compounds the. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. Web can sulfur make 6 bonds? There is a quick way to work out how many covalent bonds an element. What.
__TOP__ How Many Covalent Bonds Can Chlorine Form
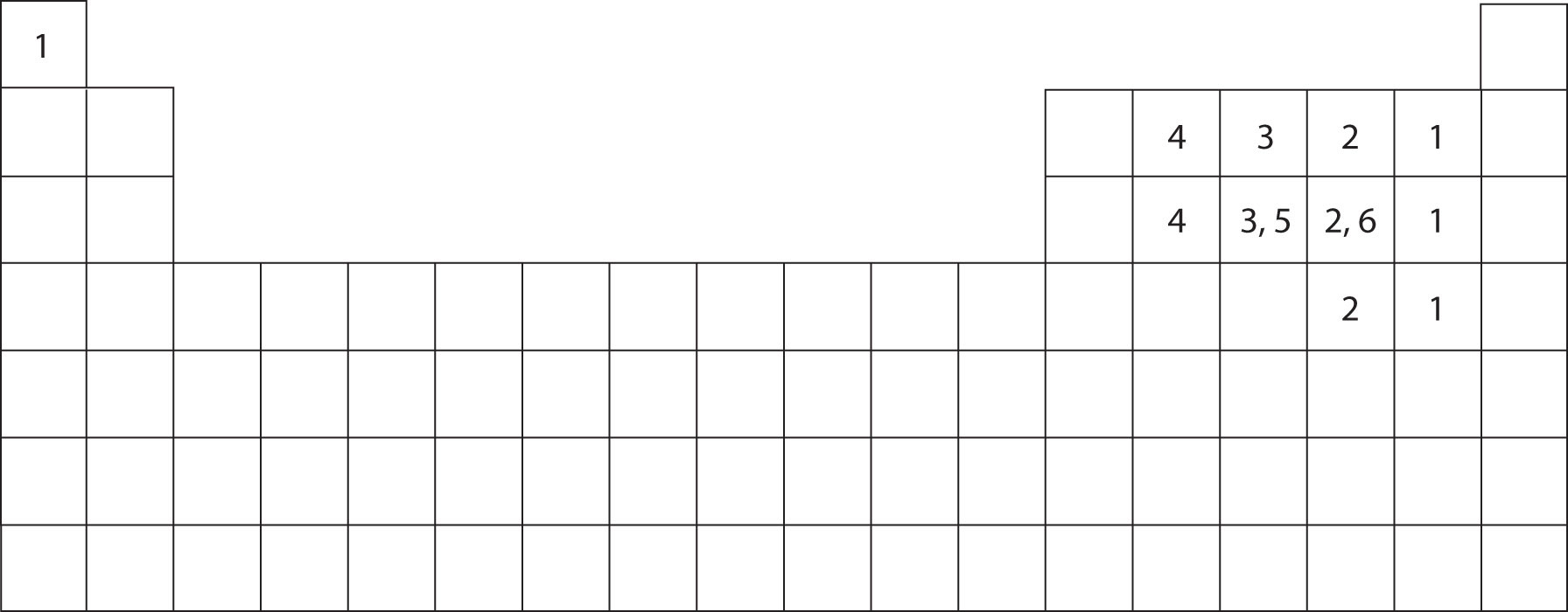
Values are given for typical oxidation number and coordination. There is a quick way to work out how many covalent bonds an element. Web up to 6% cash back how many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule? Web atoms of different elements. Web how many covalent bonds can.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Values are given for typical oxidation number and coordination. Its valency is only −2 − 2, it only needs two electrons, yet here it's getting 6 6. The same thing happens with phosphate. What is covalent bond ? Web the nucleus of a sulfur atom wil have 16 neutron and two hydrogen atoms can form covalent bond with sulfur.
Molecules Free FullText Thiosulfoxide (Sulfane) Sulfur New
The key to this problem is that electrons in. In general, sulfur can form covalent bonds by sharing its valence electrons with other atoms. Web why does sulfur form so many covalent bonds. Web how many bonds should be attached to sulfur? Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total.
Will Form Either One, Two, Three Or Four Covalent Bonds With Other Atoms.
Web each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the phosphorus's unpaired electrons to. Web how many bonds should be attached to sulfur? Web up to 6% cash back how many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule? Web sulfur can form two covalent bonds as in h2s, and can form 6 as in so3.
Web Covalent Radius Half Of The Distance Between Two Atoms Within A Single Covalent Bond.
In order to obey the octet rule, it needs. Web the maximum number of covalent bonds sulfur can form is two. Values are given for typical oxidation number and coordination. It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds.
Web How Many Covalent Bonds Can Sulfur Form?
Web most of the time a sulfur atom can form two bonds. Its valency is only −2 − 2, it only needs two electrons, yet here it's getting 6 6. A covalent bond is an. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the.
In General, Sulfur Can Form Covalent Bonds By Sharing Its Valence Electrons With Other Atoms.
Covalent bonds are formed when two non metallic elements combine by sharing their electrons. The key to this problem is that electrons in. Web can sulfur make 6 bonds? Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell.