How To Draw A Covalent Bond
How To Draw A Covalent Bond - Draw a valid electron dot structure for each of the given elements. Web a covalent bond is formed by two atoms sharing a pair of electrons. How can i draw a lewis structure of a compound? Single and multiple covalent bonds. Web we begin our discussion of the relationship between structure and bonding in covalent compounds by describing the interaction between two identical neutral atoms—for example, the h 2 molecule, which contains a purely covalent bond. What is a covalent bond? The concept of covalent bonding ties in with the octet rule. Web and introductory video for my general chemistry class (goes along with notes packet #9) showing lewis dot structures for simple molecules involving single, d. There are different kinds of covalent bonds: Here is a video summary of how you can use lewis structures to draw covalent bonds.
Web covalent bonding occurs when pairs of electrons are shared by atoms. Web draw lewis structures for covalent compounds. Web a covalent bond is formed by two atoms sharing a pair of electrons. A covalent bond is a bond formed when two atoms share electrons. Type of covalent bond formed. Octet rule and covalent bonding. Only the electrons in the outer shell take part in the bonding. (recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. There are different kinds of covalent bonds: Memorize numerical prefixes used in covalent nomenclature.
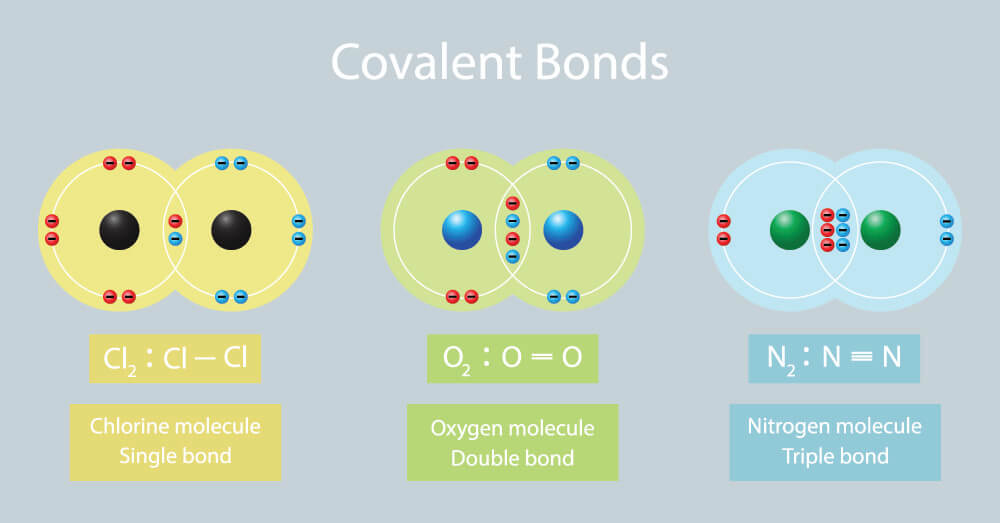
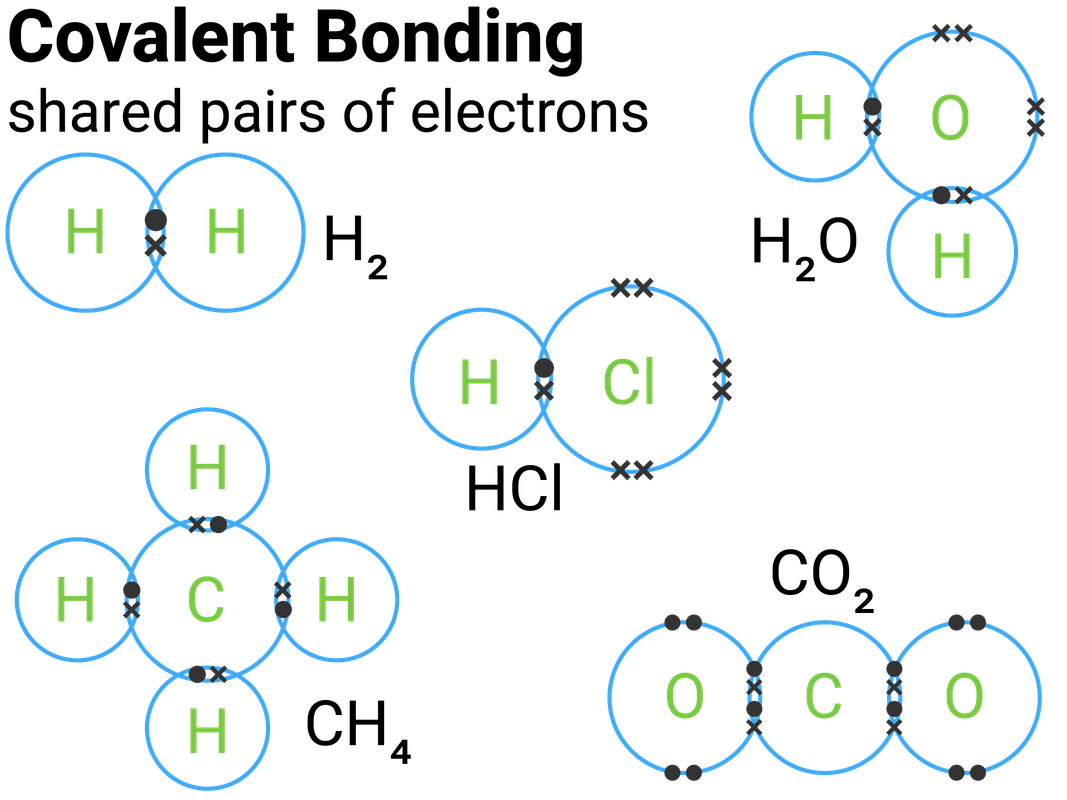
Hydrogen (h 2 ), chlorine (cl 2 ), oxygen (o 2 ), nitrogen (n 2 ), hydrogen chloride (hcl), water (h 2 o), ammonia (nh 3) and methane (ch 4) the correct dot and cross diagrams for these molecules are shown below: Web to know what types of elements bond to form covalent compounds. A single covalent bond is when two atoms share a single pair of electrons. The video covers the basic lewis structures you'll see in an introductory chemistry class. Add together the valence electrons from each atom. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web this crash course chemistry video tutorial explains the main concepts between ionic bonds found in ionic compounds and polar & nonpolar covalent bonding foun. Web draw lewis structures for covalent compounds. Single and multiple covalent bonds. Octet rule and covalent bonding.
How is a covalent bond formed
The electrons involved are in the outer shells of the atoms. Type of covalent bond formed. Determine the total number of valence electrons in the molecule or ion. Web resources for your classroom. The following procedure can be used to construct lewis electron structures for more complex molecules and ions.
Covalent Bonding The Science and Maths Zone
(recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. Web students will learn to draw covalent bonds using lewis dot structures and must incorporate their knowledge of the periodic table, bonding, and the octet rule. Memorize numerical prefixes used in covalent nomenclature. Web covalent bonding occurs when pairs of.
How To Draw Covalent Bonds
Web a covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. What is a covalent bond? Understand that covalent compound subscripts are never reduced. Add together the valence electrons from each atom.
Introducing Covalent Bonding Montessori Muddle
There are different kinds of covalent bonds: What is a covalent bond? The electrons involved are in the outer shells of the atoms. How can i draw a lewis structure of a compound? Memorize numerical prefixes used in covalent nomenclature.
How To Draw A Covalent Bond Form
What is a covalent bond? Web to know what types of elements bond to form covalent compounds. The electrons involved are in the outer shells of the atoms. There are different kinds of covalent bonds: The atoms are held together because the electron pair is attracted by both of the nuclei.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
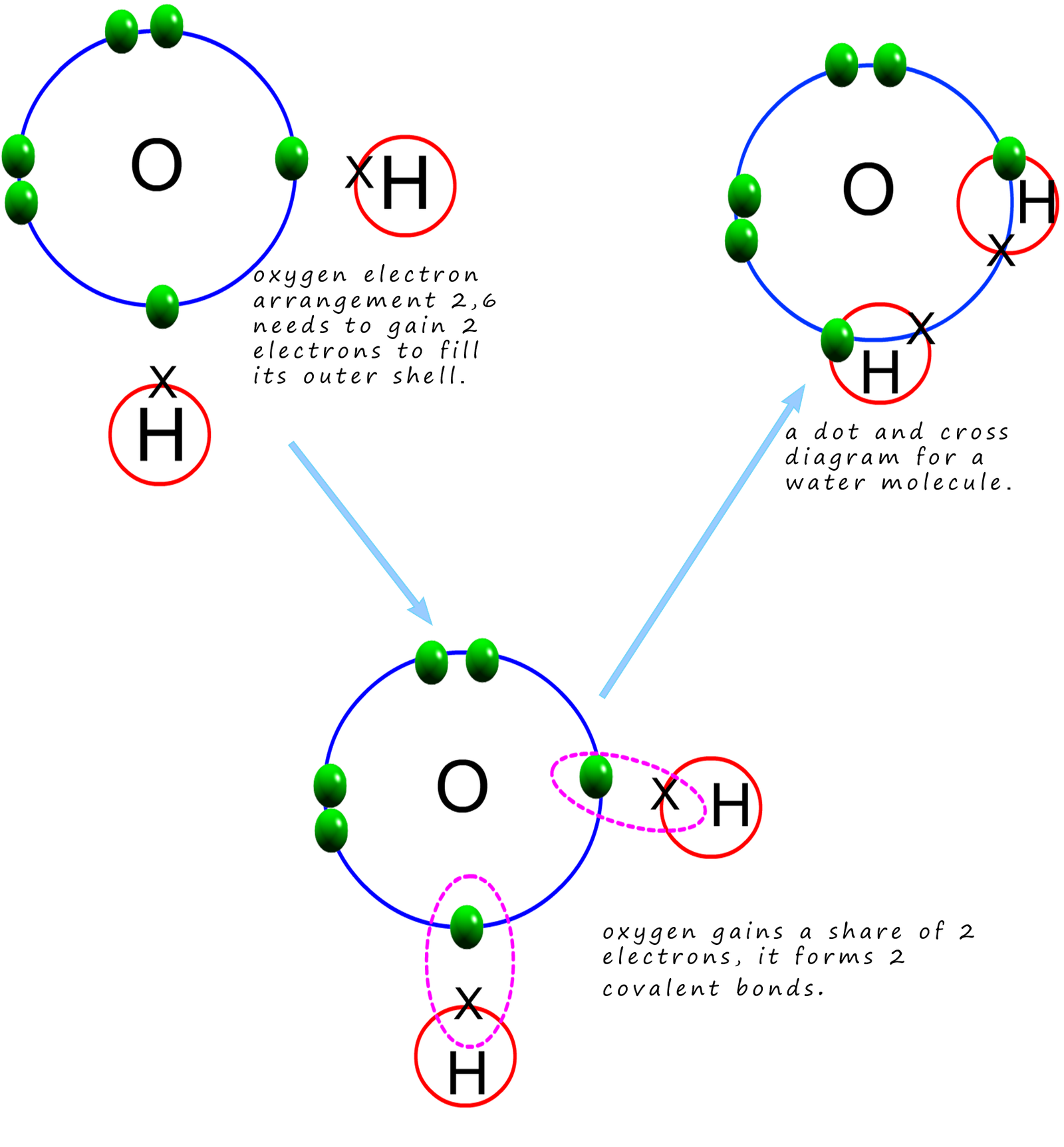
A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web the oxygen atom shares one electron with each of the two hydrogen atoms, forming two covalent bonds. Only the electrons in the outer shell take part in the bonding. Many elements found in nature exist as molecules. To appreciate how atoms share their.
Covalent Bonding The Science and Maths Zone
Web a covalent bond is formed by two atoms sharing a pair of electrons. The electrons involved are in the outer shells of the atoms. The concept of covalent bonding ties in with the octet rule. Web covalent bonding occurs when pairs of electrons are shared by atoms. Several socratic answers give the procedure.
Covalent bond (covalency) and its type Overall Science
Web to know what types of elements bond to form covalent compounds. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Number of electron pairs shared. Web we begin our discussion of the relationship between structure and bonding in covalent compounds by describing the interaction between two identical neutral atoms—for example, the.
The Covalent Bond CK12 Foundation
What is a covalent bond? Many elements found in nature exist as molecules. A covalent bond is a bond formed when two atoms share electrons. Understand that covalent compound subscripts are never reduced. Web and introductory video for my general chemistry class (goes along with notes packet #9) showing lewis dot structures for simple molecules involving single, d.
Covalent bonding
Web chemistry covalent bonds drawing lewis structures. This bonding allow atoms to have full outer shell of electrons. Web draw lewis structures for covalent compounds. A single covalent bond is when two atoms share a single pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei.
The Video Covers The Basic Lewis Structures You'll See In An Introductory Chemistry Class.
1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams, including:. Draw a valid electron dot structure for each of the given elements. The electrons involved are in the outer shells of the atoms. Number of electron pairs shared.
Here Is A Video Summary Of How You Can Use Lewis Structures To Draw Covalent Bonds.
This bonding allow atoms to have full outer shell of electrons. Web this crash course chemistry video tutorial explains the main concepts between ionic bonds found in ionic compounds and polar & nonpolar covalent bonding foun. Web a covalent bond is formed by two atoms sharing a pair of electrons. The following procedure can be used to construct lewis electron structures for more complex molecules and ions.
Arrange The Atoms To Show Specific Bonds.
A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web students will learn to draw covalent bonds using lewis dot structures and must incorporate their knowledge of the periodic table, bonding, and the octet rule. Memorize numerical prefixes used in covalent nomenclature. Type of covalent bond formed.
Web Resources For Your Classroom.
What is a covalent bond? Octet rule and covalent bonding. Web covalent bonding occurs when pairs of electrons are shared by atoms. The atoms are held together because the electron pair is attracted by both of the nuclei.