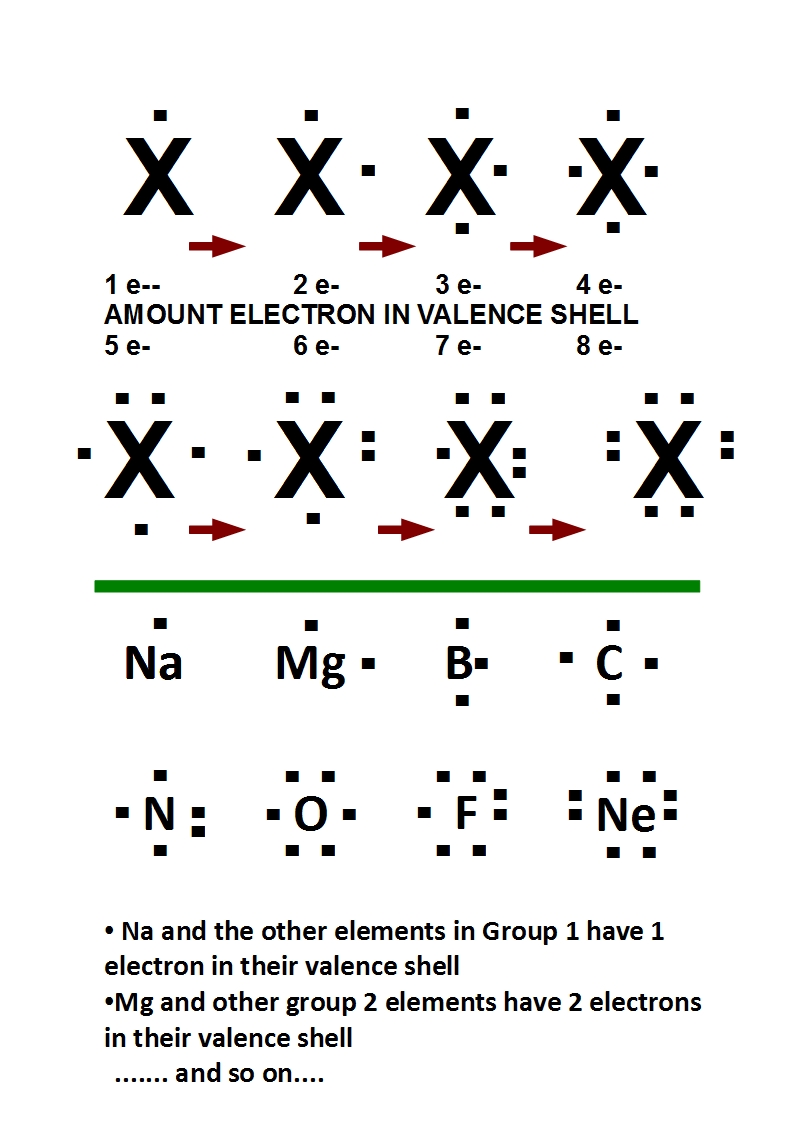
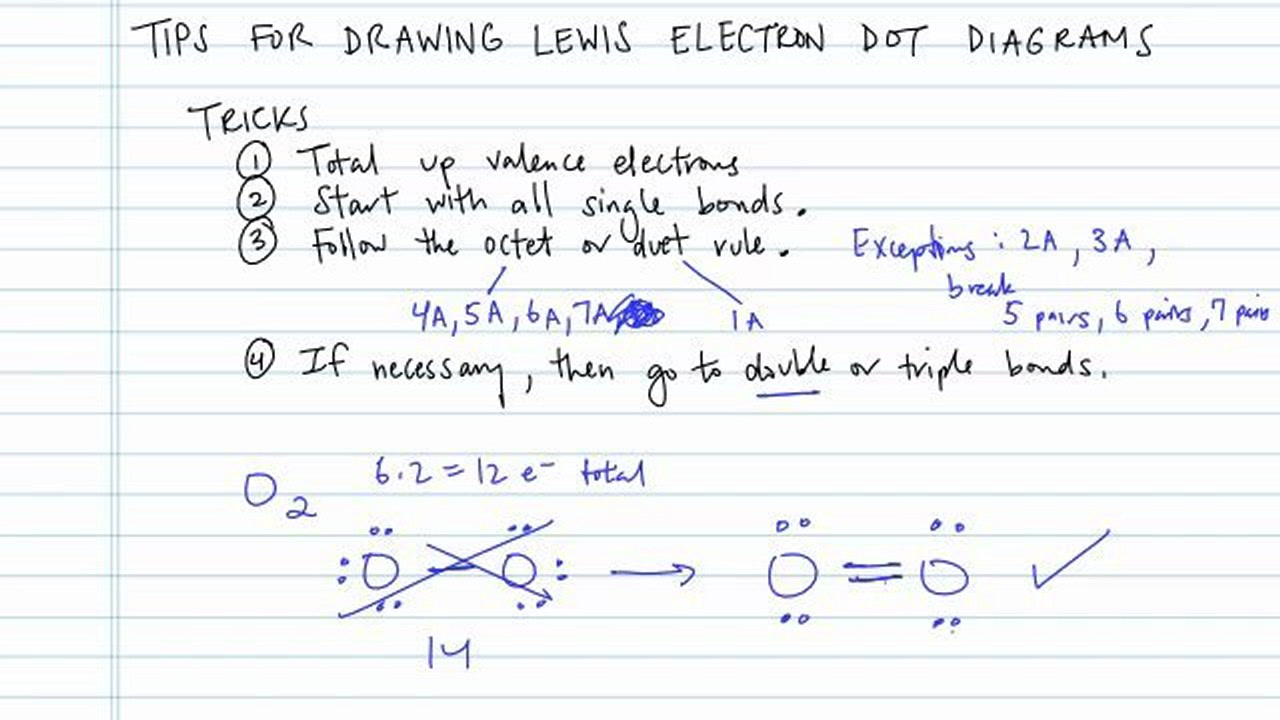
How To Draw An Electron Dot Diagram
How To Draw An Electron Dot Diagram - Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. Lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Draw a lewis electron dot diagram for an atom or a monatomic ion. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. A lewis structure can be drawn for any. The number of dots equals the number of valence electrons in the atom.
First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of. The steps that must be followed while drawing a lewis structure are listed below. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. These dots are arranged to the right and left and above. The example is for the nitrate ion. A beryllium atom, with two valence electrons, has the electron dot diagram below: Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom.
Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The steps that must be followed while drawing a lewis structure are listed below. A lewis structure can be drawn for any. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Draw a lewis electron dot diagram for an atom or a monatomic ion. Lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of.
How to Draw a Lewis Structure
These dots are arranged to the right and left and above and below the symbol. The steps that must be followed while drawing a lewis structure are listed below. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. A lewis structure can be drawn for any. The number of dots.
Lewis Electron Dot Structure Calculator
Web a lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons.
BohrRutherford Diagrams & Lewis Dot Diagrams Eve Wongworakul
Connect the atoms to each other with single bonds to form a “skeleton structure.”. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Lewis structures (also known as lewis dot diagrams, electron.
rules for drawing lewis dot structures for molecules
A lewis electron dot diagram (or electron dot. A lewis structure can be drawn for any. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. A beryllium atom, with two valence electrons, has the electron dot diagram below: Web a lewis electron dot structure describes the bonding.
3 Ways to Draw Lewis Dot Structures wikiHow
Web a lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms. The steps that must be followed while drawing a lewis structure are listed below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are..
How To Draw Electron Dot Diagrams Elementchampionship Jeffcoocctax
A lewis structure can be drawn for any. Web here are the steps to draw a lewis structure. Draw a lewis electron dot diagram for an atom or a monatomic ion. A lewis electron dot diagram (or electron dot. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are.
How To Draw Lewis Dot Structures » Doubleprogram
A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Connect the atoms to each other with single bonds to form a “skeleton structure.”. The number of dots equals the number of valence electrons in the atom. The number of dots equals the number of.
How To Draw An Electron Dot Diagram Photos Cantik
A lewis structure can be drawn for any. Web so we're going to a lot more examples for drawing dot structures in the next several videos, and see how drawing dot structures allows you to predict the shapes of different molecules. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they.
How To Draw Electron Dot Diagrams Elementchampionship Jeffcoocctax
Web electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures).
3 Ways to Draw Lewis Dot Structures wikiHow
Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Draw a lewis electron dot diagram for an atom or a monatomic ion. The number of dots equals the number of valence electrons in the atom..
In Almost All Cases, Chemical Bonds Are Formed By Interactions Of Valence Electrons In Atoms.
Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. The steps that must be followed while drawing a lewis structure are listed below. These dots are arranged to the right and left and above and below the symbol.
Draw A Lewis Electron Dot Diagram For An Atom Or A Monatomic Ion.
Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web here are the steps to draw a lewis structure. Lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
First, The Total Number Of Valence Electrons Present In The Molecule Is Calculated By Adding The Individual Valencies Of.
The example is for the nitrate ion. The number of dots equals the number of valence electrons in the atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. These dots are arranged to the right and left and above.
When Constructing A Lewis Diagram, Keep In Mind The Octet Rule, Which Refers To The Tendency Of Atoms To Gain, Lose, Or Share.
Web so we're going to a lot more examples for drawing dot structures in the next several videos, and see how drawing dot structures allows you to predict the shapes of different molecules. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. A beryllium atom, with two valence electrons, has the electron dot diagram below:
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)