How To Draw Hydrogen Bonding
How To Draw Hydrogen Bonding - Web hydrogen bonds between water molecules give water its high boiling point, high heat capacity, and surface tension. There are various ways of drawing this and you will need to be familiar with all of them. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. Web water molecules forming hydrogen bonds with one another. Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water. Web these relatively powerful intermolecular forces are described as hydrogen bonds. Does chlorine form hydrogen bonds? Web exercise \ (\pageindex {1}\) how many hydrogens in figure \ (\pageindex {1}\) can form hydrogen bonds? Web so, when you're drawing a bond line structure and you have a carbon chain you wanna show that carbon chain in a zig zag pattern.
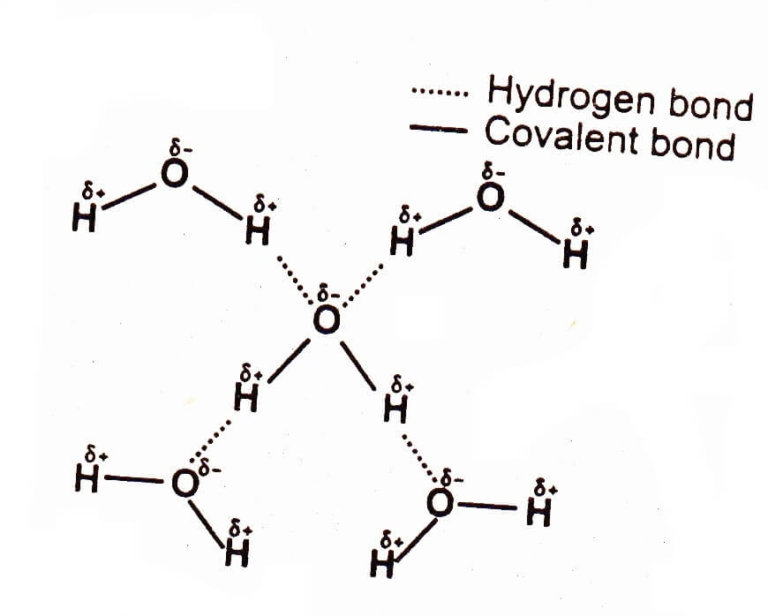
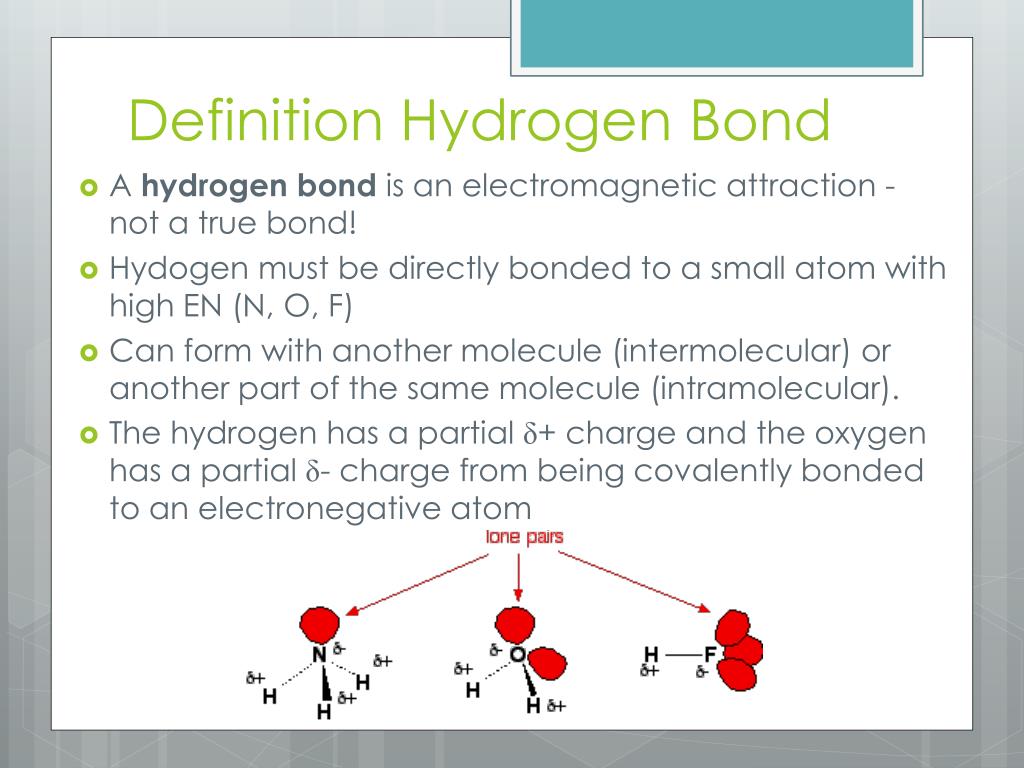
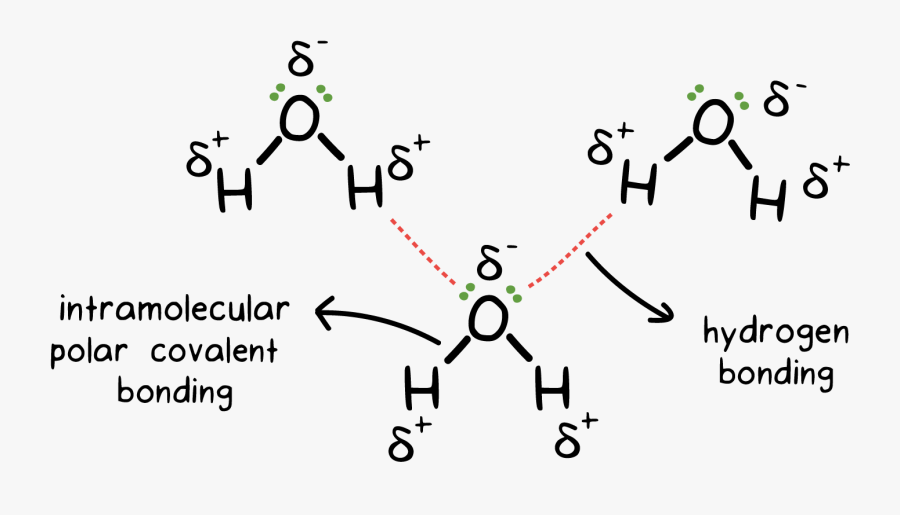
Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. Web learn how to study the hydrogen bond in chemical systems using avogadro. The number of hydrogen bonds depends on: Each water molecule is hydrogen bonded to four others. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Two fluorine atoms can form a molecule of f 2 in the same fashion. So hydrogen is never a true central atom of a molecule simply because its limited in its bonding abilities. Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch. Web exercise \ (\pageindex {1}\) how many hydrogens in figure \ (\pageindex {1}\) can form hydrogen bonds?
Web for example, two hydrogen atoms can form a bond, producing a molecule of h 2. Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web water molecules forming hydrogen bonds with one another. 10k views 3 years ago #exploding #teacher #chemical. Ammonia can form a maximum of one hydrogen bond per molecule. Hydrogen that is bonded to very electronegative elements (n, o and f) will have a big δ+ (partial positive charge). Web learn which types of molecules make hydrogen bonds and how to draw the hydrogen bonding interactions between two molecules of the same type and between two m. Only one, the one at the very top which is attached to the highly electrongative oxygen atom (red), all the others are attached to carbon and can not hydrogen bond.
[DIAGRAM] Labeled Diagram Of Hydrogen Bonding
Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); Web a single hydrogen atom has one valence electron, so it only needs to react with one other atom covalently with a single bond to get a second electron. Web water as a perfect example of hydrogen bonding. Next, let's think about the carbon hydrogen bonds. Hydrogen bonds are strong intermolecular.
How To Draw Hydrogen Bonds
Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web learn how to study the hydrogen bond in chemical systems using avogadro. Web for example, two hydrogen atoms can form a bond, producing a molecule of h 2. A structural formula shows how the various atoms are bonded..
How To Draw Hydrogen Bonds Askworksheet
Web learn how to study the hydrogen bond in chemical systems using avogadro. The origin of hydrogen bonding. The number of hydrogen bonds depends on: A structural formula shows how the various atoms are bonded. The number of hydrogen atoms attached to o or n in the molecule.
Hydrogen Bonding American Chemical Society
Each water molecule is hydrogen bonded to four others. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. The partial negative charge on the o of.
Hydrogen Bonding Chemistry Skills
The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. Two fluorine atoms can form a molecule of f 2 in the same fashion. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch. Web a single hydrogen atom.
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
The origin of hydrogen bonding. There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. The number of lone pairs on the o or n. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Does chlorine form hydrogen bonds?
H2o Drawing Chemical Bond Intermolecular Hydrogen Bonding In Water
Web these relatively powerful intermolecular forces are described as hydrogen bonds. Web c5h12 + 8o2 → 5co2 + 6h2o (1) (1) c 5 h 12 + 8 o 2 → 5 c o 2 + 6 h 2 o. So hydrogen is never a true central atom of a molecule simply because its limited in its bonding abilities. The number.
Hydrogen Bonding in water Dr. M. Chemistry Tutor
Web these relatively powerful intermolecular forces are described as hydrogen bonds. There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. In cases like this, the bonding in the organic molecule isn't important. Note that each atom must contribute one electron to the bond. A structural.
Diagram Of Water Molecules Hydrogen Bonding
So hydrogen is never a true central atom of a molecule simply because its limited in its bonding abilities. Next, let's think about the carbon hydrogen bonds. The origin of hydrogen bonding. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. Web exercise \ (\pageindex {1}\) how many hydrogens in figure.
Draw a diagram of water molecules, labeling the hydrogen bond and
The number of hydrogen bonds depends on: There are various ways of drawing this and you will need to be familiar with all of them. Two fluorine atoms can form a molecule of f 2 in the same fashion. Web learn which types of molecules make hydrogen bonds and how to draw the hydrogen bonding interactions between two molecules of.
Each Water Molecule Is Hydrogen Bonded To Four Others.
Web these relatively powerful intermolecular forces are described as hydrogen bonds. This is why the boiling point of water is higher than. Web the force of attraction, shown here as a dotted line, is called a hydrogen bond. Hydrogen bonding between different parts of the same chain (intramolecular bonding;
Web Water Molecules Forming Hydrogen Bonds With One Another.
Next, let's think about the carbon hydrogen bonds. Using lewis structures, we can represent this as follows: The molecules which have this extra bonding are: 4.7k views 7 years ago 1d:
There Are Exactly The Right Numbers Of + Hydrogens And Lone Pairs So That Every One Of Them Can Be Involved In Hydrogen Bonding.
Web for example, two hydrogen atoms can form a bond, producing a molecule of h 2. This video shows three examples of drawing for the formation of hydrogen bond. Atoms can form more than one bond. Two fluorine atoms can form a molecule of f 2 in the same fashion.
Does Chlorine Form Hydrogen Bonds?
Only one, the one at the very top which is attached to the highly electrongative oxygen atom (red), all the others are attached to carbon and can not hydrogen bond. A structural formula shows how the various atoms are bonded. Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. Hydrogen that is bonded to very electronegative elements (n, o and f) will have a big δ+ (partial positive charge).
![[DIAGRAM] Labeled Diagram Of Hydrogen Bonding](http://ffden-2.phys.uaf.edu/webproj/211_fall_2016/Roger_Vang/Roger_Vang/Hydrogen Bonding.png)