How To Draw Ionic Bonding
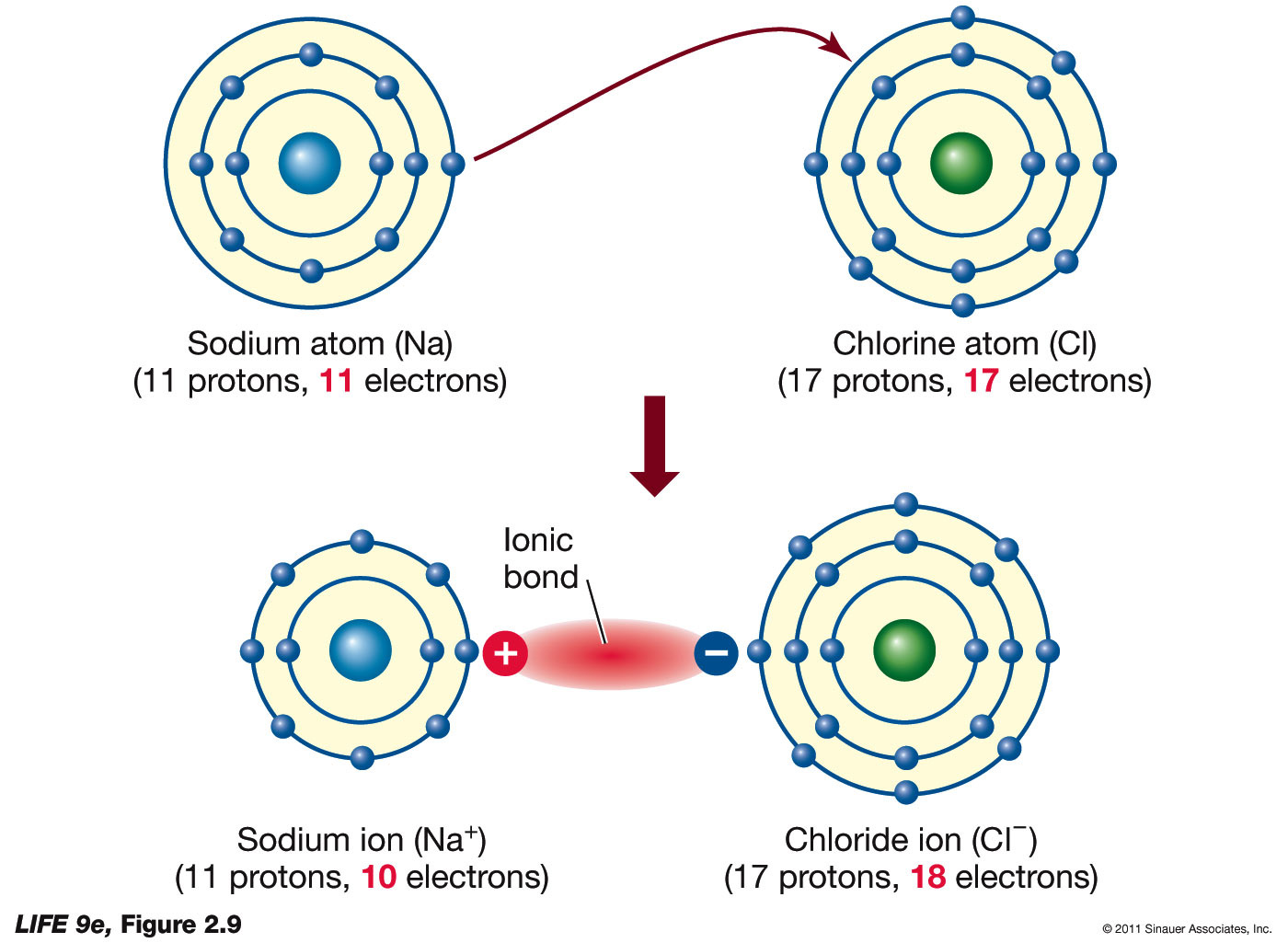
How To Draw Ionic Bonding - Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. These oppositely charged ions attract each other to form ionic networks (or lattices). Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds. Web lewis structures are mostly applied to covalent molecules, and while it is exceedingly uncommon you should do the same for ionic compounds. 166k views 11 years ago chemical equations; During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. This chemistry video explains how to draw the lewis structures of ionic compounds. At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance.
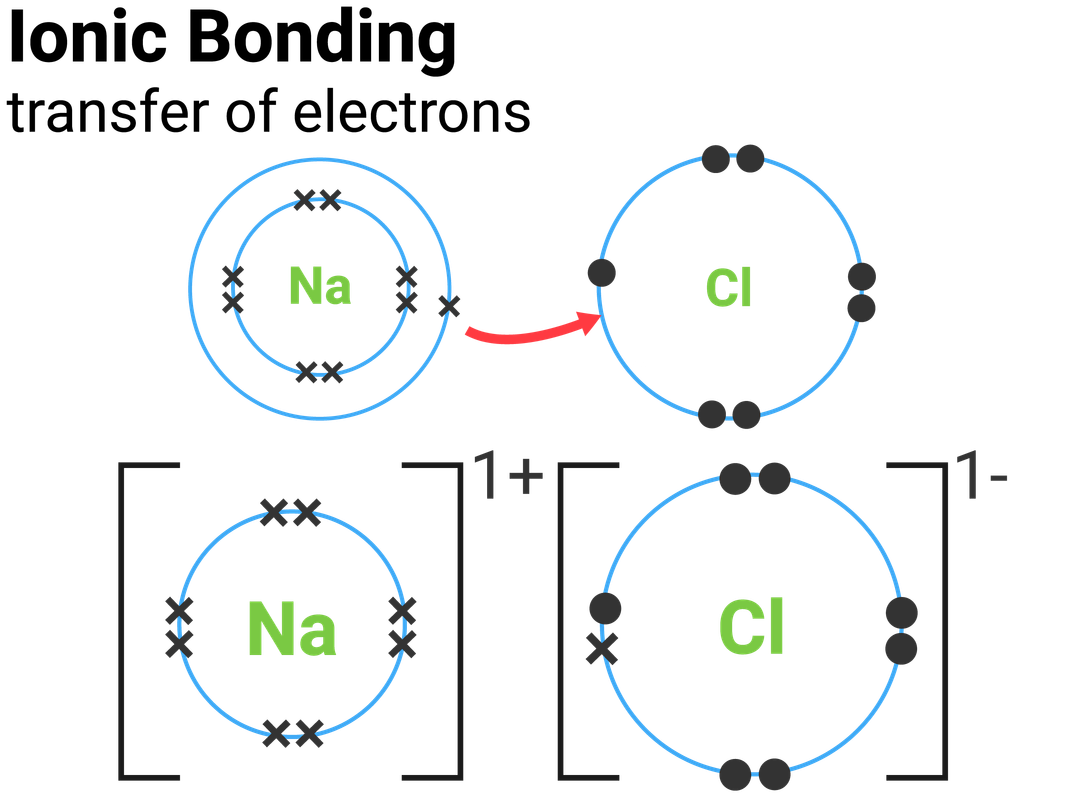
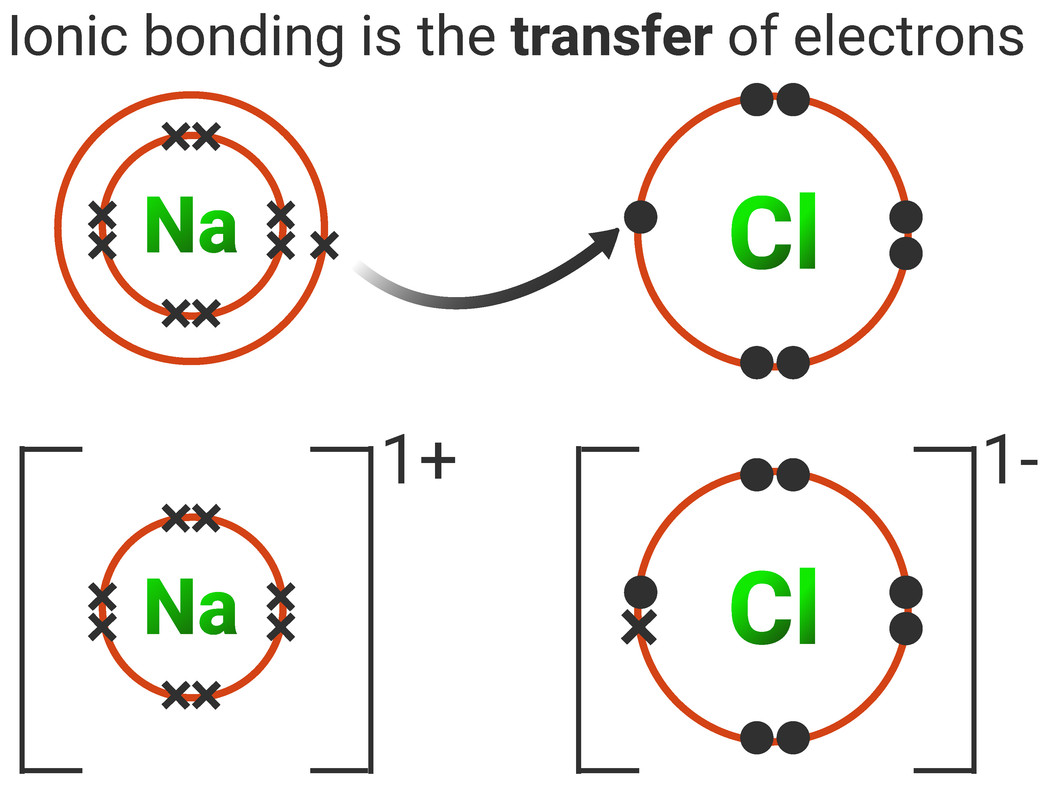
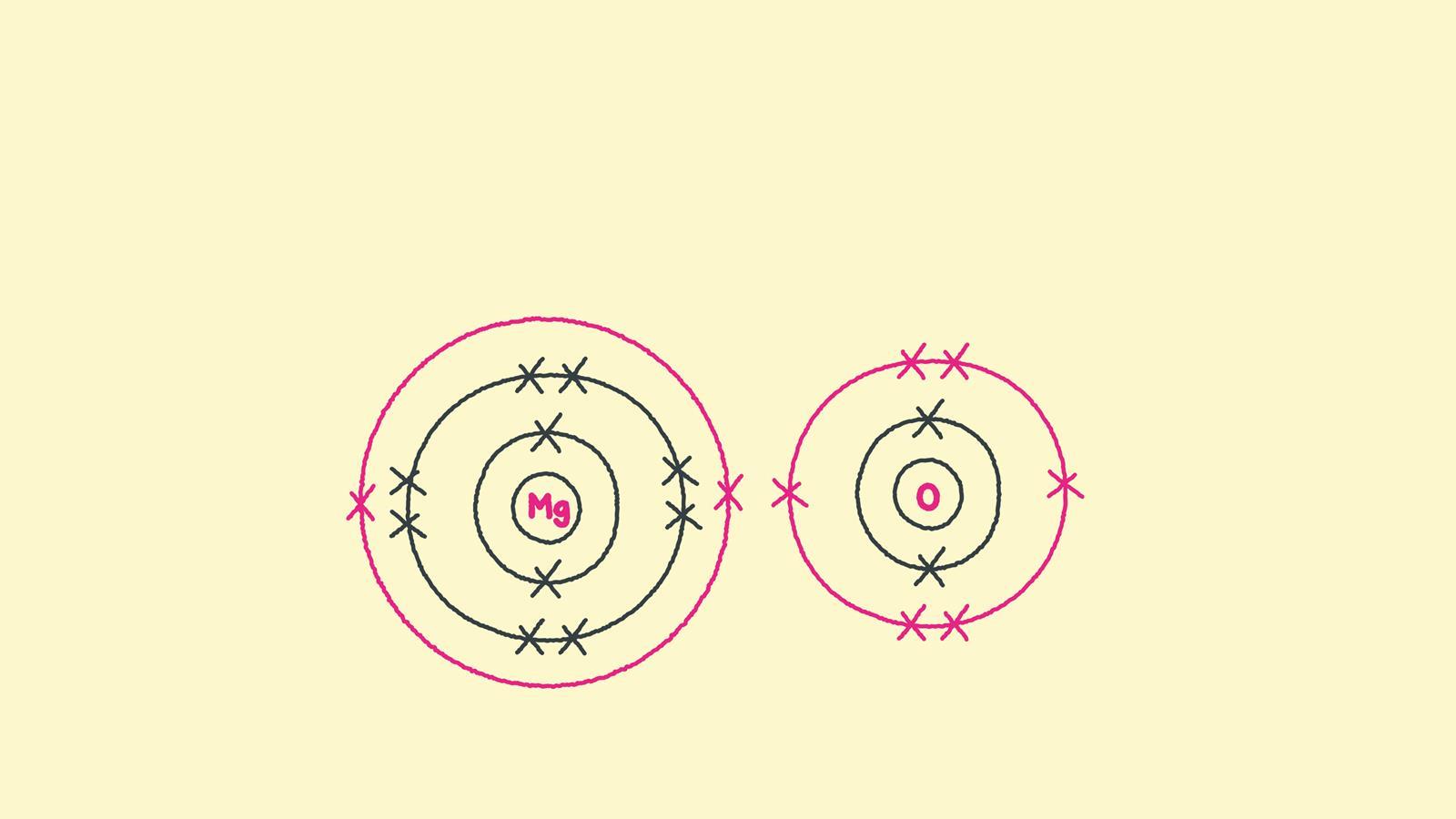
(note that we denote ions with brackets around the structure, indicating the. Swap the crosses for dots in one of your diagrams. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web how to draw the lewis structures of ionic compounds. Magnesium oxide dot & cross diagram. Web we summarize the important points about ionic bonding: A dot and cross diagram models the transfer of electrons from metal. Web draw the electron configuration diagram for each atom. Web draw dot and cross diagrams of ionic bonding and explain how an ionic lattice is held together. These oppositely charged ions attract each other to form ionic networks (or lattices).
While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: This chemistry video explains how to draw the lewis structures of ionic compounds. Ionic bonds are caused by electrons transferring from one atom to another. State the limitations of a range of models used to represent ionic bonding. 6.7k views 7 years ago edexcel. The astute reader may have noticed something: Draw the outer shell of each atom. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web we summarize the important points about ionic bonding:
Ionic Bonding GCSE Science Chemistry Get To Know Science YouTube
Web draw the electron configuration diagram for each atom. Web lewis structures are mostly applied to covalent molecules, and while it is exceedingly uncommon you should do the same for ionic compounds. Draw the electron configuration diagrams for each atom in the compound. (note that we denote ions with brackets around the structure, indicating the. State the limitations of a.
IGCSE Chemistry 2017 1.40 Draw DotandCross Diagrams to Show the
For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. Web draw dot and cross diagrams of ionic bonding and explain how an ionic lattice is held together. It's just for ionic compounds electrons aren't shared so.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
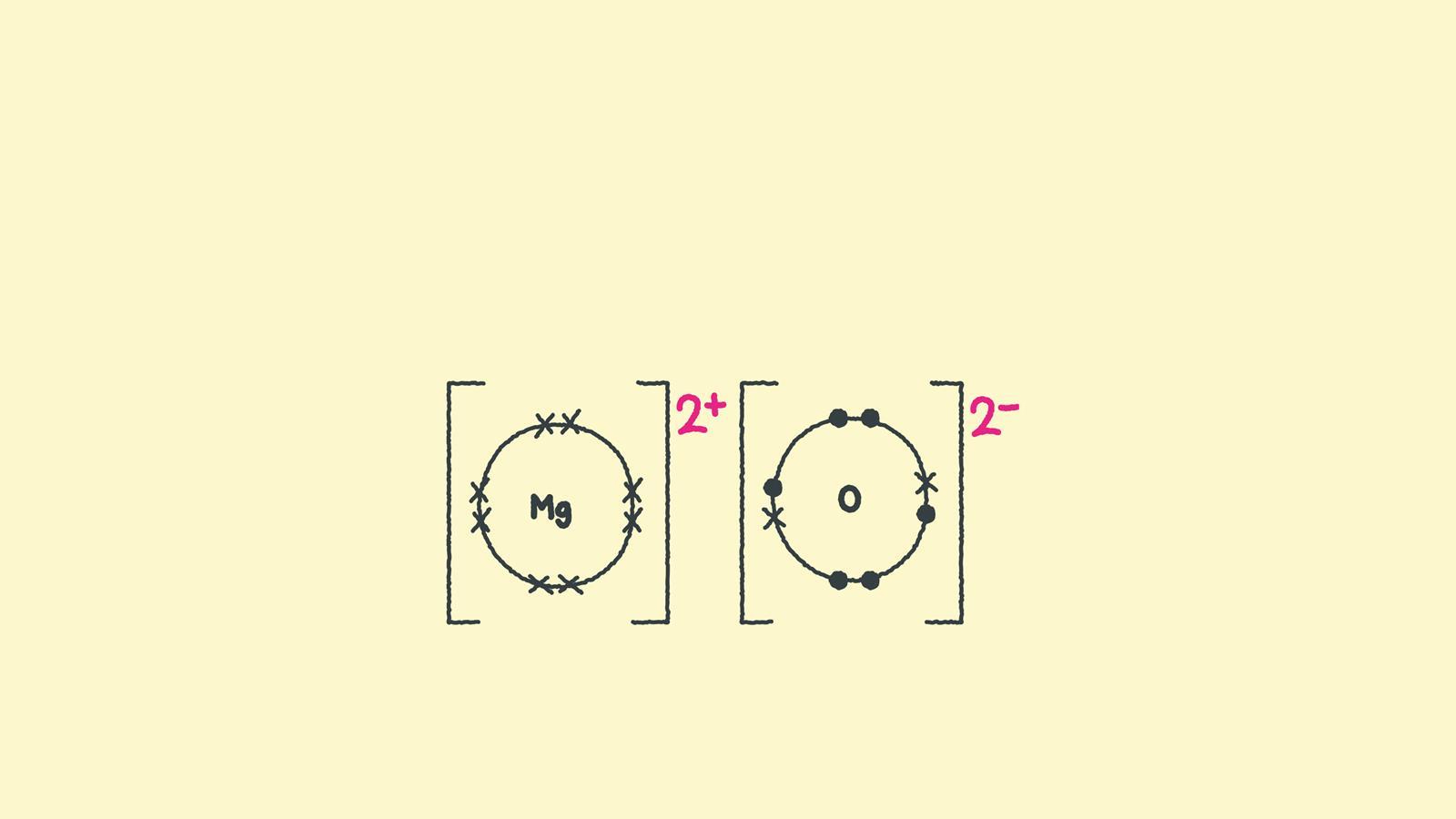
Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds. Draw the electron configuration diagram for each atom. Magnesium has two electrons in its outer shell, oxygen has six..
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
(note that we denote ions with brackets around the structure, indicating the. Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds. Web draw dot and cross diagrams of ionic bonding and explain how an ionic lattice is held together. Electrostatics explains why this happens:.
How to draw ionic bonding dot and cross diagrams Feature RSC Education
Magnesium has two electrons in its outer shell, oxygen has six. Magnesium oxide dot & cross diagram. These grades are the stepping stone to your future. While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: Many of the ions that form have eight electrons in their valence.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
These oppositely charged ions attract each other to form ionic networks (or lattices). Ionic bonds are caused by electrons transferring from one atom to another. Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. In almost all cases, chemical bonds are formed by interactions.
ionic bond Definition, Properties, Examples, & Facts Britannica
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. When drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for. By losing those electrons, these metals can achieve noble gas configuration and satisfy the octet rule. Swap the crosses for shell. Opposite charges.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
This chemistry video explains how to draw the lewis structures of ionic compounds. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Draw the outer shell of each atom. At r 0 ,.
How to draw ionic bonding dot and cross diagrams Feature RSC Education
Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. These oppositely charged ions attract each other to form ionic networks (or lattices). When oppositely charged ions are brought together from r = ∞ to r = r.
Ionic bond Science, Chemistry, Chemical Bonds ShowMe
The astute reader may have noticed something: At r 0 , the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Draw the electron configuration diagrams for each atom in the compound. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful..
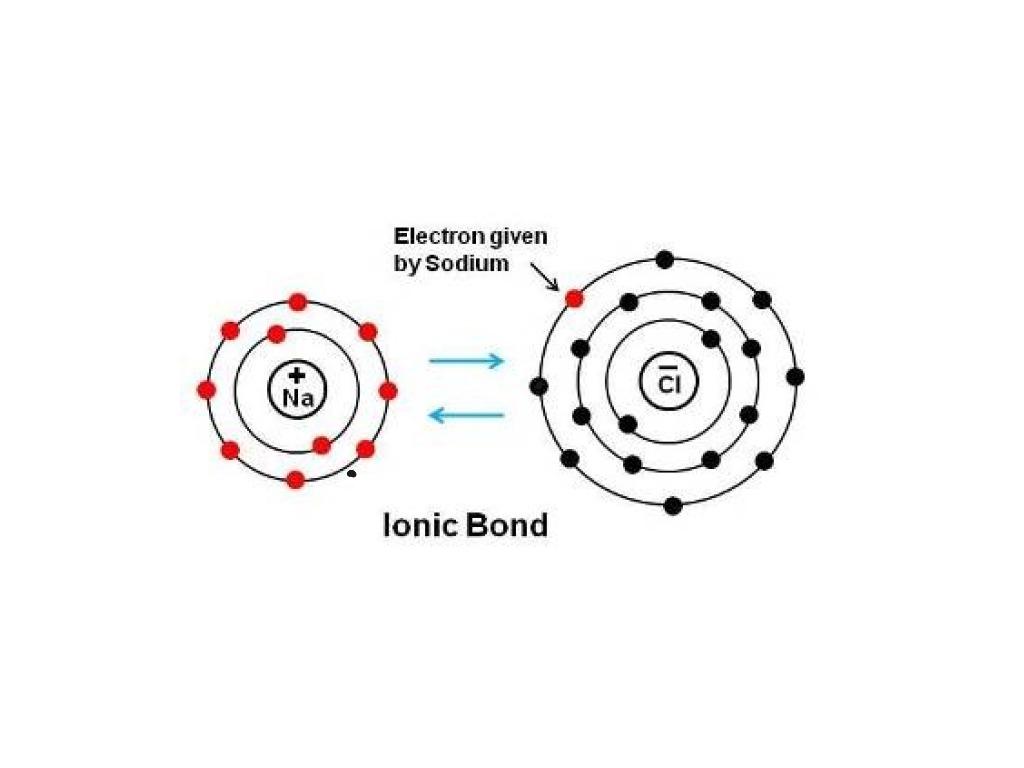
Work Out How Many Electrons Need To Be Transferred.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Web how to draw the lewis structures of ionic compounds.
Web Ionic Bonds Require An Electron Donor, Often A Metal, And An Electron Acceptor, A Nonmetal.
Examples include nacl, mgf2, k2o, and al2o3.how. Draw the electron configuration diagram for each atom. These grades are the stepping stone to your future. A dot and cross diagram models the transfer of electrons from metal.
Web Lewis Structures Are Mostly Applied To Covalent Molecules, And While It Is Exceedingly Uncommon You Should Do The Same For Ionic Compounds.
Draw the outer shell of each atom. It's just for ionic compounds electrons aren't shared so you won't have things like single bonds between atoms. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Swap the crosses for dots in one of your diagrams.
At R 0 , The Ions Are More Stable (Have A Lower Potential Energy) Than They Are At An Infinite Internuclear Distance.
Web we summarize the important points about ionic bonding: Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: Web i want to help you achieve the grades you (and i) know you are capable of;