How To Draw Mo Diagrams
How To Draw Mo Diagrams - Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). N* = # electrons in antibonding orbitals. Molecular orbitals for a simple pi bond; Draw the mo diagram for `o_2^+` this is a bit of a curveball, but a perfectly valid problem. This approach is used only when the group orbitals are not obvious by inspection. Determine point group of molecule (if linear, use d. Two for the allyl cation, three for the allyl radical, and four for the allyl anion. H has a 1s orbital. F has a 2s orbital and 3 2p orbitals (x,y,z). In the event of a discrepancy, the official drawing results shall prevail.
We need to know what orbitals we are using. “ the only way to make sense of change is to plunge into it, move with it, and join the dance. Determine the total number of valence electrons in the he 2 2 + ion. 2h 2v d ∞h or c ∞v) (z axis is principal axis; Determine point group of molecule (if linear, use d. F has a 2s orbital and 3 2p orbitals (x,y,z). The f 2s is nonbonding. `o_2^+` is just the ionized form of `o_2`; Construct a qualitative molecular orbital diagram for chlorine, cl 2. N* = # electrons in antibonding orbitals.
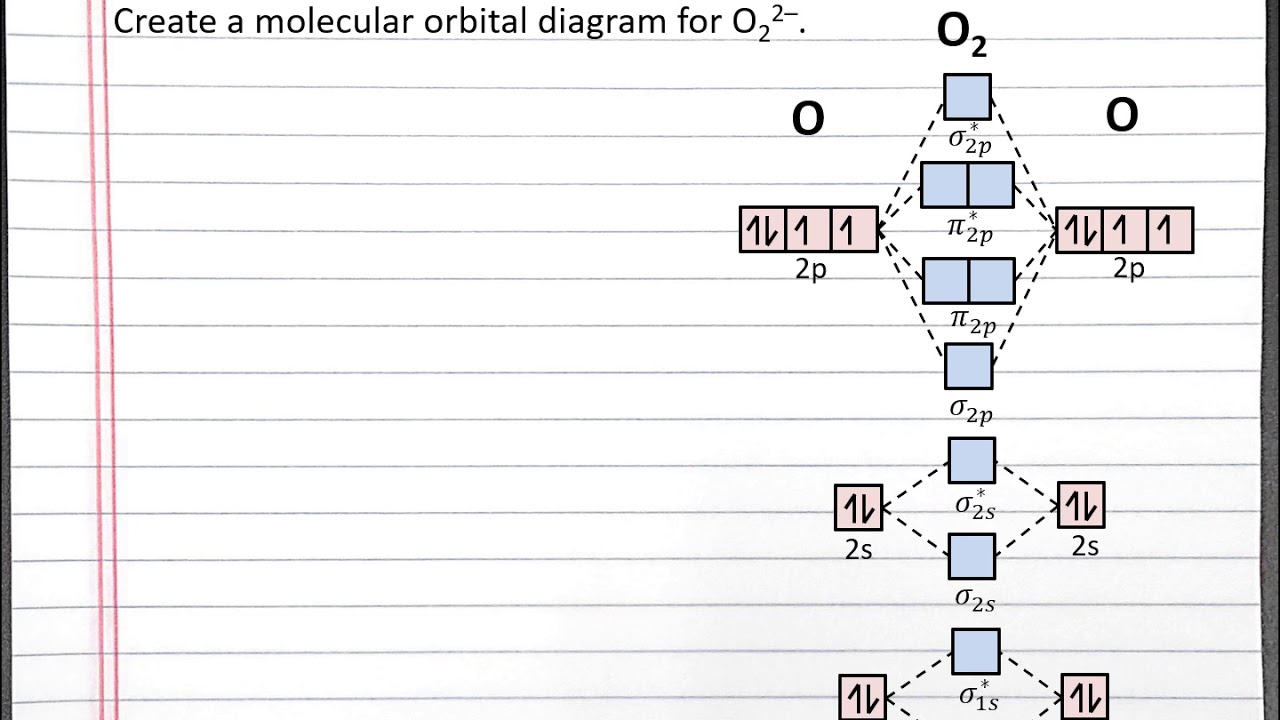
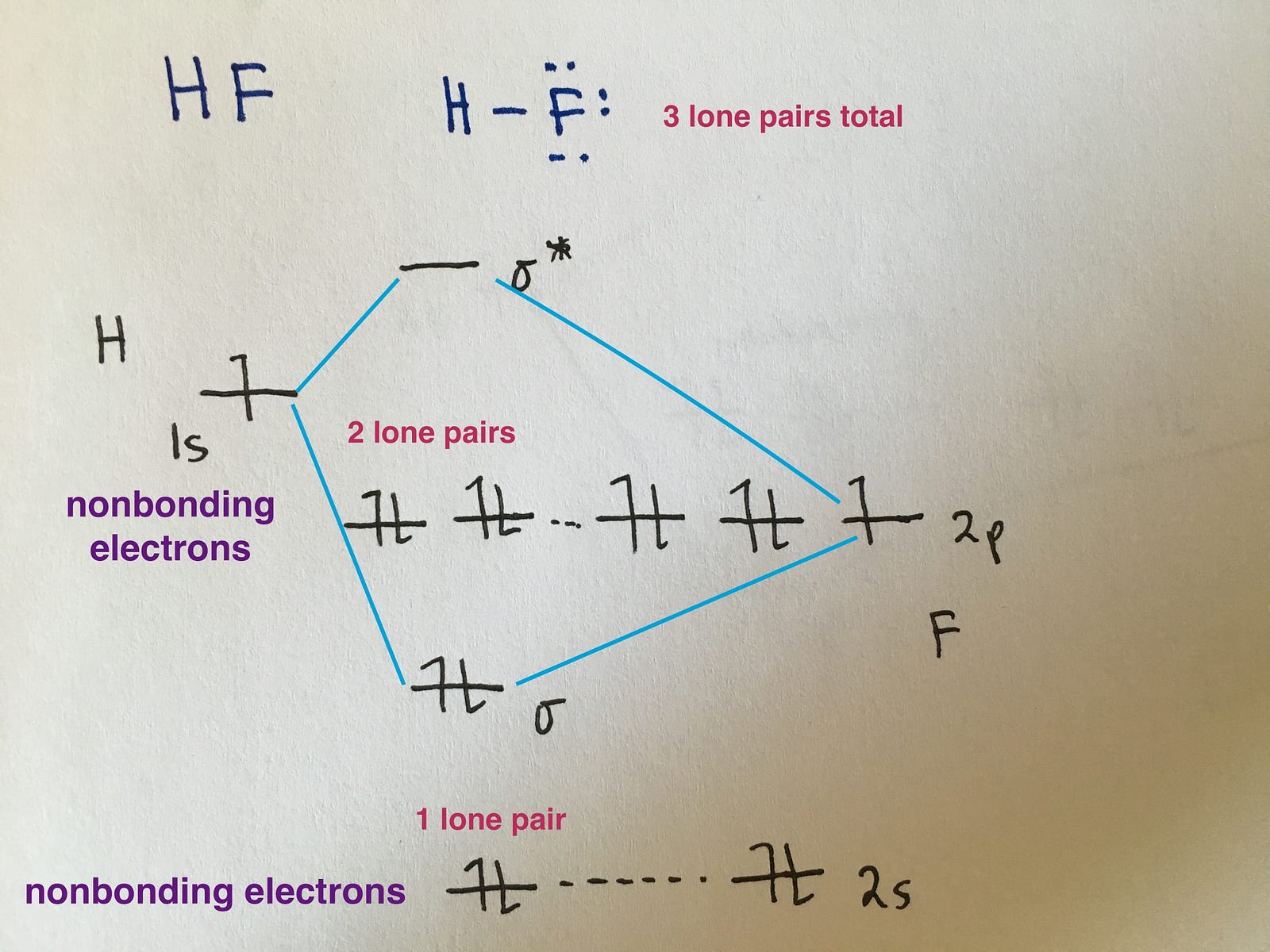
Determine the total number of valence electrons in the he 2 2 + ion. Web combine the two he valence atomic orbitals to produce bonding and antibonding molecular orbital; Draw the mo for o 2: That is, it's `o_2` with `1` missing electron. H has a 1s orbital. Determine point group of molecule (if linear, use d. F has a 2s orbital and 3 2p orbitals (x,y,z). Web example 1 1: Web similar to atomic orbitals, we can write electron configuration energy diagrams for molecular orbitals (figure 9.20 “ hydrogen molecular orbital electron configuration energy diagram”).notice that the atomic orbitals of each atom are written on either side, and the newly formed molecular orbitals are written in the centre of the diagram. Web mo diagram for hf.
how to draw molecular orbital diagrams for polyatomic molecules
Draw the mo for o 2: Assign x, y, z coordinates and c instead of. Greater overlap = greater change in. Web mo diagram for hf. Try the following mo's on your own, and then check with the answers provided.
How To Draw A Molecular Orbital Diagram Elevatorunion6
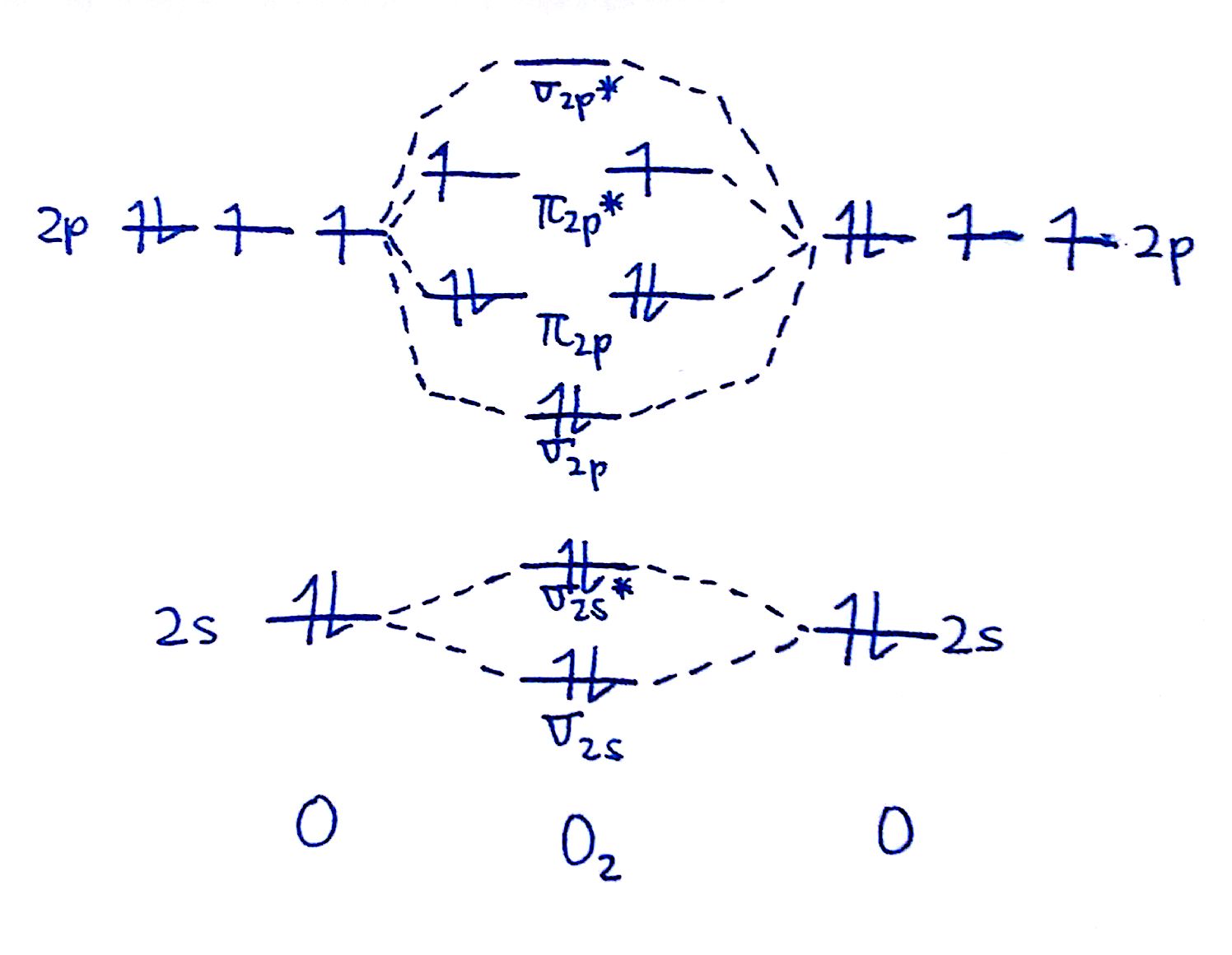
We will predict their bond order and see how the energies of the different orbitals change. This diagram is based on calculations and comparison to experiments. As a rule of thumb, a bond order = 1 equates to a single bond, a bond order = 2 equates to a double bond, etc. N* = # electrons in antibonding orbitals. 2h.
MO Diagram Overview, How to draw MO Diagram and Solved example along
2h 2v d ∞h or c ∞v) (z axis is principal axis; It describes the formation of bonding and antibonding molecular o. Web for more complicated molecules, it is better to use the procedure given earlier: The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. `o_2^+` is just the ionized form of.
MO Diagrams
Web similar to atomic orbitals, we can write electron configuration energy diagrams for molecular orbitals (figure 9.20 “ hydrogen molecular orbital electron configuration energy diagram”).notice that the atomic orbitals of each atom are written on either side, and the newly formed molecular orbitals are written in the centre of the diagram. N = # electrons in bonding orbitals. Web this.
How to Draw Molecular Orbital Diagrams (MO DIAGRAMS) Explanation YouTube
Construct a qualitative molecular orbital diagram for chlorine, cl 2. The best way to learn how to draw mo diagrams is to work on practice problems. Web how to draw molecular orbital diagrams for conjugated systems Determine point group of molecule (if linear, use d. Official winning numbers are those selected in the respective drawings and recorded under the observation.
How To Draw Mo Diagram Wiring Site Resource
`o_2^+` is just the ionized form of `o_2`; Based on the amount of orbital overlap, the relative changes in energy differ going from the atomic orbital to the molecular orbital. Web how to draw molecular orbital diagrams for conjugated systems Recall that a cation indicates a loss of `1` electron. The mo diagram will be the same as the mo.
Molecular Orbital Diagrams simplified by Megan Lim Medium
Now let's put these ideas together to make an mo diagram for hf. Official winning numbers are those selected in the respective drawings and recorded under the observation of an independent accounting firm. This approach is used only when the group orbitals are not obvious by inspection. Based on the amount of orbital overlap, the relative changes in energy differ.
Molecular Orbital Diagrams simplified by Megan Lim Medium
Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). That is, it's `o_2` with `1` missing electron. Construct a qualitative molecular orbital diagram for chlorine, cl 2. The f 2s is nonbonding. The mo diagram will be.
MO Diagrams
N* = # electrons in antibonding orbitals. We need to know what orbitals we are using. It also illustrates the operations and attributes of the classes. They are usually used to explore domain concepts, understand software requirements and. This diagram is based on calculations and comparison to experiments.
MO Diagram Overview, How to draw MO Diagram and Solved example along
Web mo diagram for hf the ao energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. It describes the formation of bonding and antibonding molecular o. The mo diagram will be the same as the mo diagram of `o_2`, except with `1` less electron. Based on the amount of orbital overlap, the relative.
Assign X, Y, Z Coordinates And C Instead Of.
The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. As a rule of thumb, a bond order = 1 equates to a single bond, a bond order = 2 equates to a double bond, etc. The f 2s is nonbonding. “ the only way to make sense of change is to plunge into it, move with it, and join the dance.
Notice That The Bonding Orbitals Are Bigger On The Oxygen And The Antibonding Orbitals Are Bigger On.
Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). The rules for building up pi molecular orbital; N = # electrons in bonding orbitals. Draw the mo for o 2:
In The Event Of A Discrepancy, The Official Drawing Results Shall Prevail.
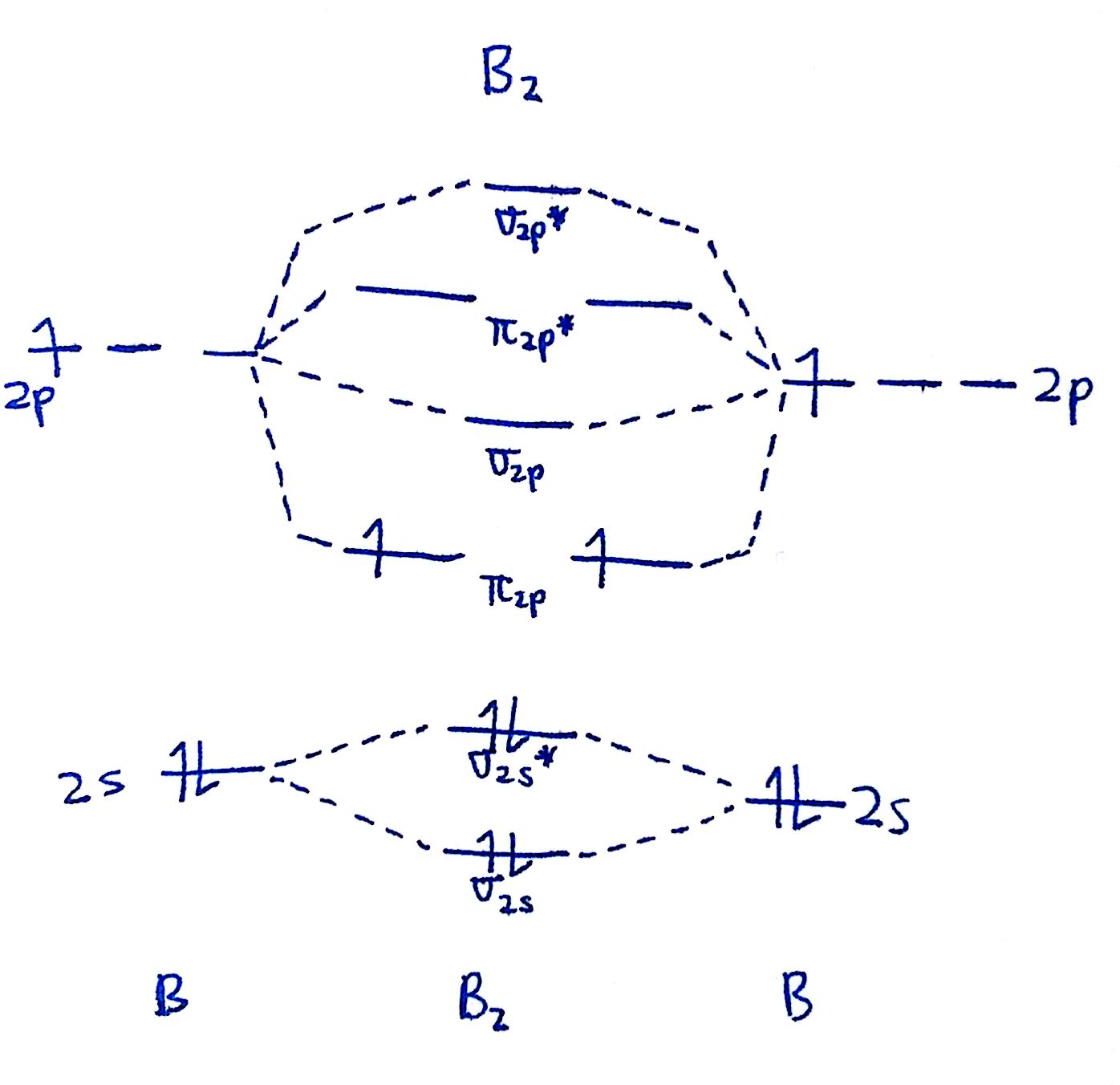
Construct a qualitative molecular orbital diagram for chlorine, cl 2. Molecular orbitals for a simple pi bond; We will predict their bond order and see how the energies of the different orbitals change. We are only going to consider valence orbitals.
Draw The Mo Diagram For `O_2^+` This Is A Bit Of A Curveball, But A Perfectly Valid Problem.
Web however, recall that the more electronegative atom will be lower on the diagram. The allyl cation, allyl radical, and allyl anion Web how to draw molecular orbital diagrams for conjugated systems Web mo diagram for hf.