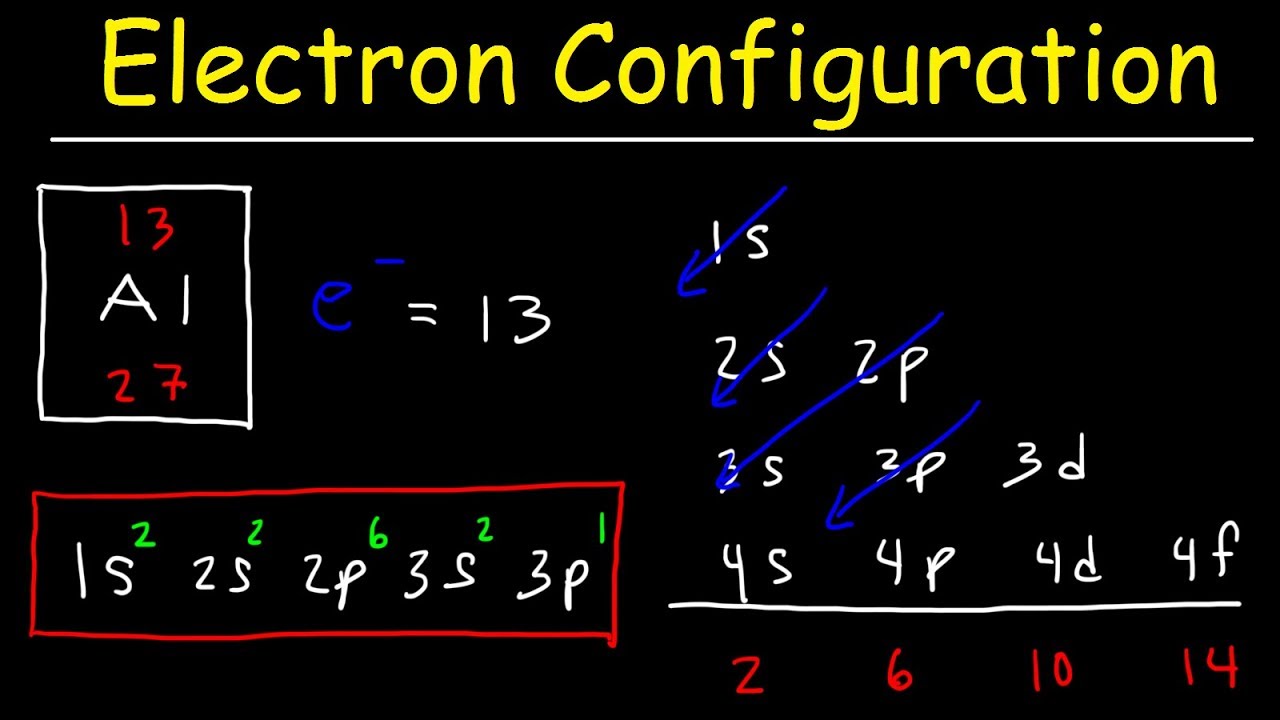
How To Draw The Electron Configuration
How To Draw The Electron Configuration - So if there's one proton there must be one electron. Web a simple tutorial on how to draw electron configurations efficiently, and how they are written. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Carbon (atomic number 6) has six electrons. Write the electron configuration for. Web electron configuration diagrams | properties of matter | chemistry | fuseschoollearn the basics about drawing electron configuration diagrams. We build electron configurations by filling the lowest energy orbitals first then filling progressively higher energy orbitals. Web to write electron configuration of an element, locate its symbol in adomah periodic table and cross out all elements that have higher atomic numbers. The helium atom contains two protons and two electrons.
Following hydrogen is the noble gas helium, which has an atomic number of 2. Web electron configuration diagrams | properties of matter | chemistry | fuseschoollearn the basics about drawing electron configuration diagrams. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. And we're gonna use the aufbau principle. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). It contains plenty of practice problems including the electron conf. Then, since the lithium ion has one less electron, remove an electron from. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web the electron configuration is a description of where electrons are in a molecule or atom. The electron configuration is the standard notation used to describe the electronic structure of an atom.
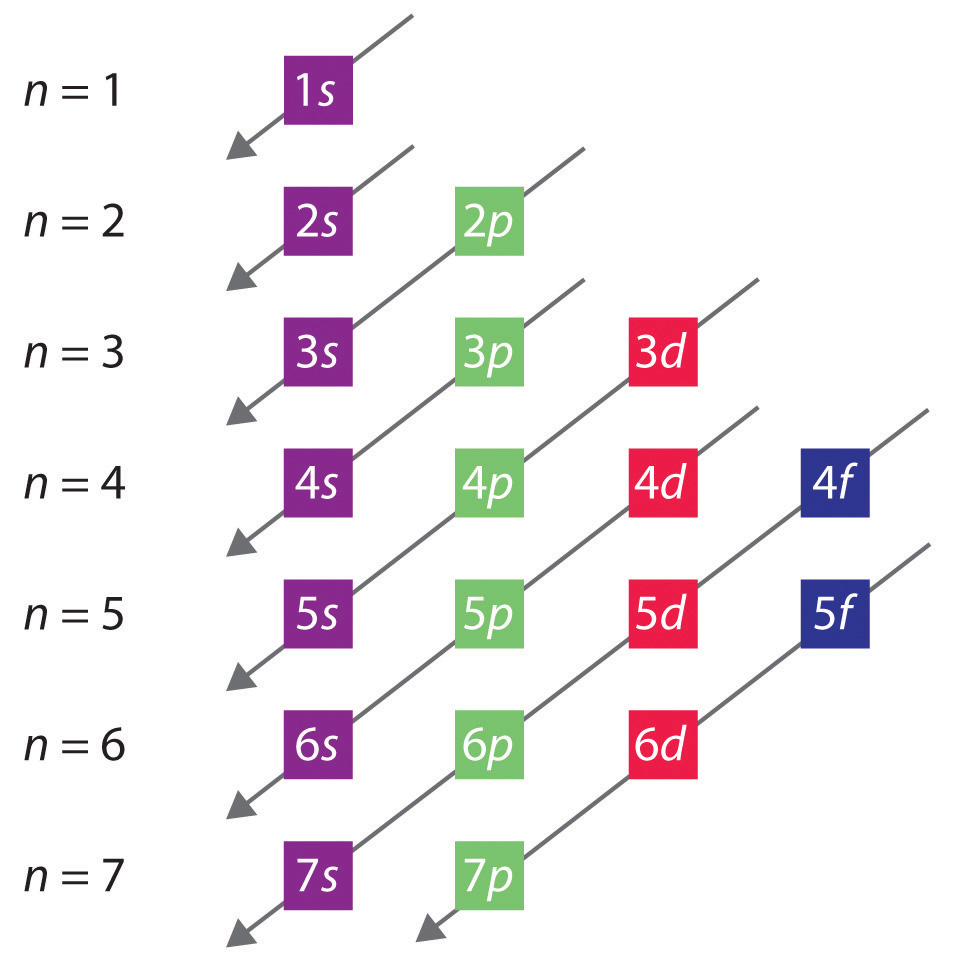
This is known as the aufbau principal. Web to write electron configuration of an element, locate its symbol in adomah periodic table and cross out all elements that have higher atomic numbers. Web electron configuration diagrams | properties of matter | chemistry | fuseschoollearn the basics about drawing electron configuration diagrams. An orbital box diagram can be written as well. Systems with a greater number of electrons will occupy a greater amount of energy levels, meaning that they also will utilize higher energy levels. To learn how to write electronic configurations, detailed explanation, filling of orbital with faqs, visit. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Aufbau is german for building up. A neutral helium atom, with an atomic number of 2 ( z = 2), has two electrons.
Drawing electron configuration diagrams Chemistry for All The Fuse
We build electron configurations by filling the lowest energy orbitals first then filling progressively higher energy orbitals. Web in a neutral atom, the number of protons is equal to the number of electrons. So our goal is to write an electron configuration for that one electron of hydrogen. A neutral helium atom, with an atomic number of 2 ( z.
Drawing electron configuration diagrams YouTube
In electronic configuration electrons are arranged in various shells, subshell and orbital by following certain rules. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. For example, if you need to write electron configuration of erbium (68), cross out elements 69 through 120. The electron configuration is the standard notation used to describe the.
Electron Configuration Definition, Examples, Chart, and Diagram
The helium atom contains two protons and two electrons. It contains plenty of practice problems including the electron conf. Electrons occupy orbitals that have characteristic levels of energy. For example, potassium has 19 electrons. Following hydrogen is the noble gas helium, which has an atomic number of 2.
Drawing electron configurations with Aufbau/orbital diagram YouTube
It contains plenty of practice problems including the electron conf. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Then, since the lithium ion has one less electron, remove an electron from. Shared pairs of electrons are drawn as lines between atoms,.
How to draw Electroninbox diagrams Electronic Configurations? [GCE A
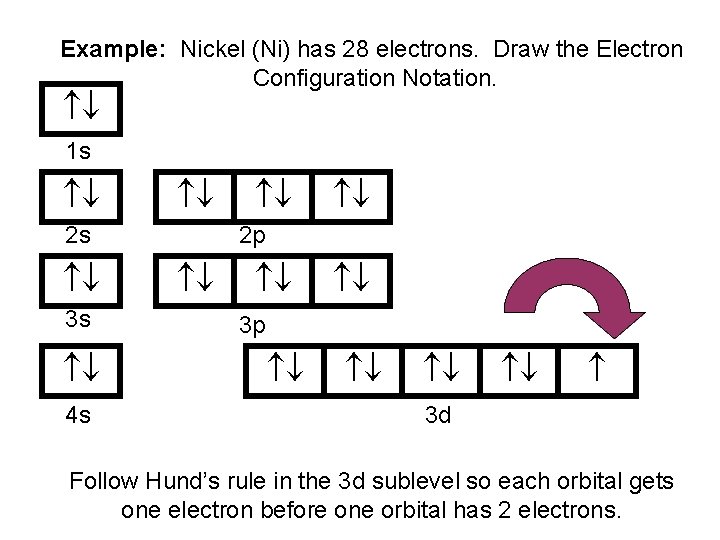
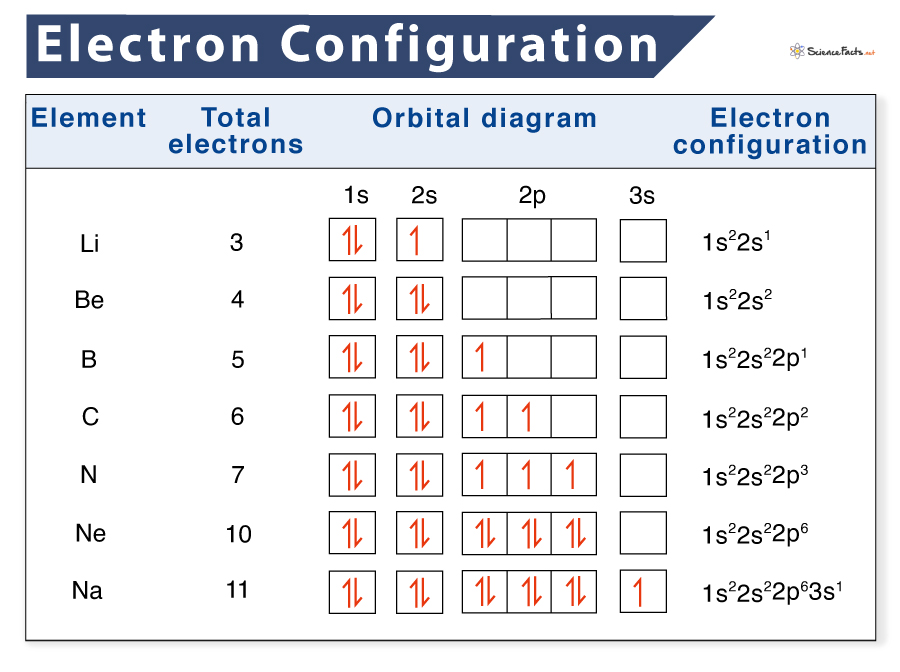
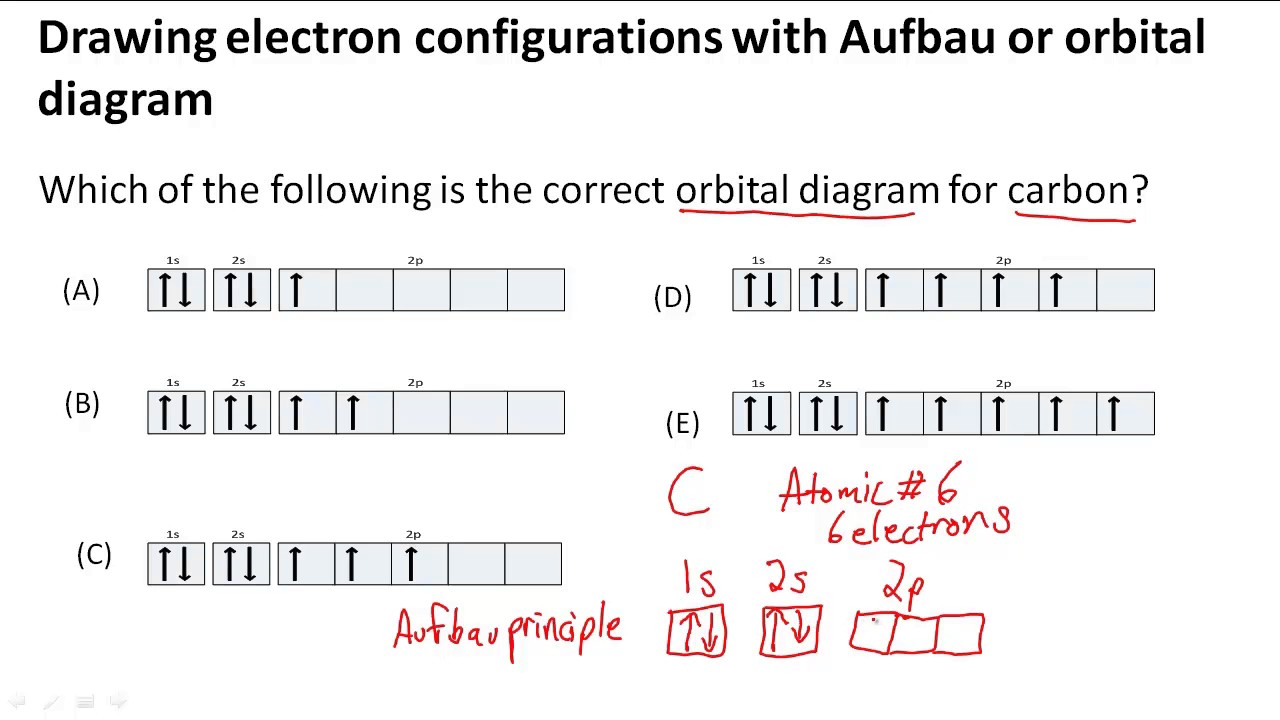
Aufbau is german for building up. The number of dots equals the number of valence electrons in the atom. Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Web to write electron configuration of an element, locate its symbol in adomah periodic table and cross out all.
2.2 Electron Configurations Chemistry LibreTexts
Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Write the electron configuration for. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). These dots are.
Electron Configuration Chart
Notice numbers 1 through 8 at the base of the table. Find the element on the periodic table. Aufbau is german for building up. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Web electron configuration diagrams | properties of matter | chemistry | fuseschoollearn the basics about drawing electron configuration.
Electron Configuration Basic introduction YoutuBeRandom
Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 6.8.1 6.8. Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Then, since the lithium ion has one less electron, remove an electron from. Web.
List of Electron Configurations of Elements
The helium atom contains two protons and two electrons. We place one electron in the orbital that is. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. Then, since the lithium ion has one less electron, remove an electron from. Web a lewis electron dot diagram (or electron.
Electron Configuration Chapter 5 Electrons have 3 levels
Web to write electron configuration of an element, locate its symbol in adomah periodic table and cross out all elements that have higher atomic numbers. Then, since the lithium ion has one less electron, remove an electron from. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web to find the electron configuration.
Electrons Occupy Orbitals That Have Characteristic Levels Of Energy.
Aufbau is german for building up. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 6.8.1 6.8. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. Web the electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells.
For Example, To Find The Configuration For The Lithium Ion (Li⁺), Start With Neutral Lithium (1S²2S¹).
For example, potassium has 19 electrons. We place one electron in the orbital that is. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. These dots are arranged to the right and left and above and below.
Web This Chemistry Video Tutorial Provides A Basic Introduction Into Electron Configuration.
Then, since the lithium ion has one less electron, remove an electron from. Systems with a greater number of electrons will occupy a greater amount of energy levels, meaning that they also will utilize higher energy levels. This is known as the aufbau principal. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.
Web When Drawing Orbital Diagrams, We Include Empty Boxes To Depict Any Empty Orbitals In The Same Subshell That We Are Filling.
It contains plenty of practice problems including the electron conf. The helium atom contains two protons and two electrons. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from. To learn how to write electronic configurations, detailed explanation, filling of orbital with faqs, visit.
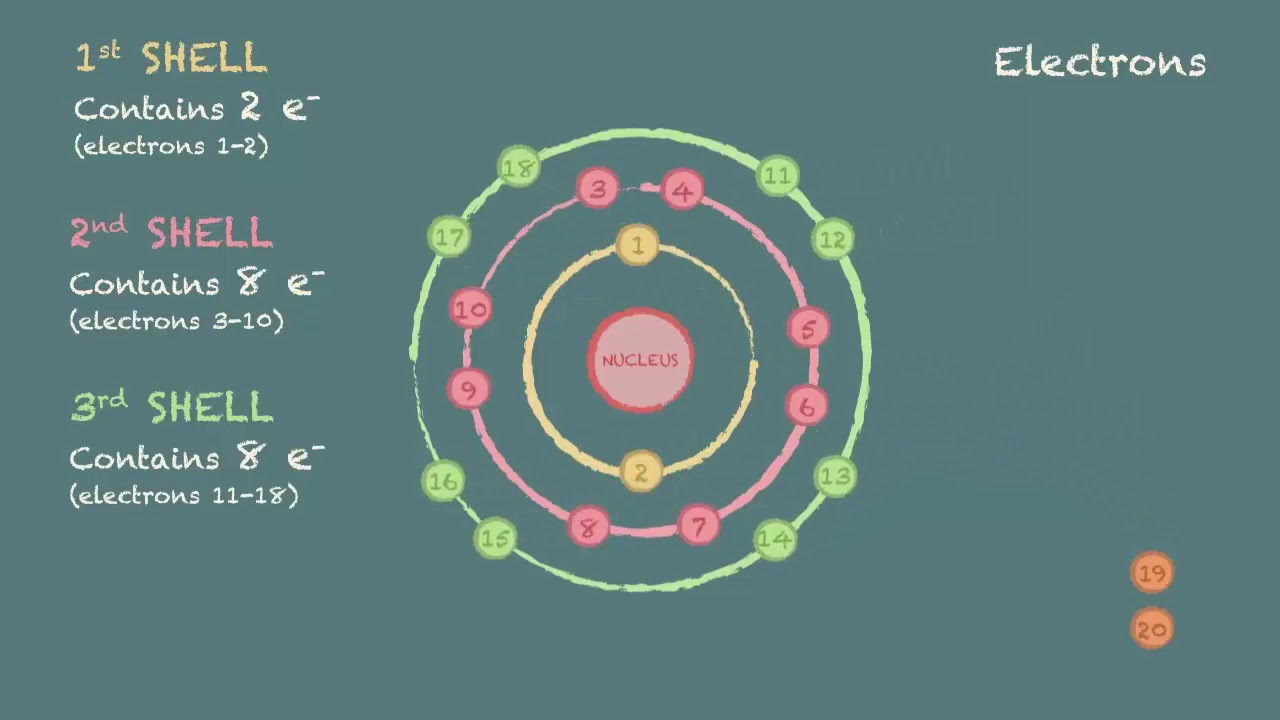

:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)