How To Know How Many Bonds An Element Can Form
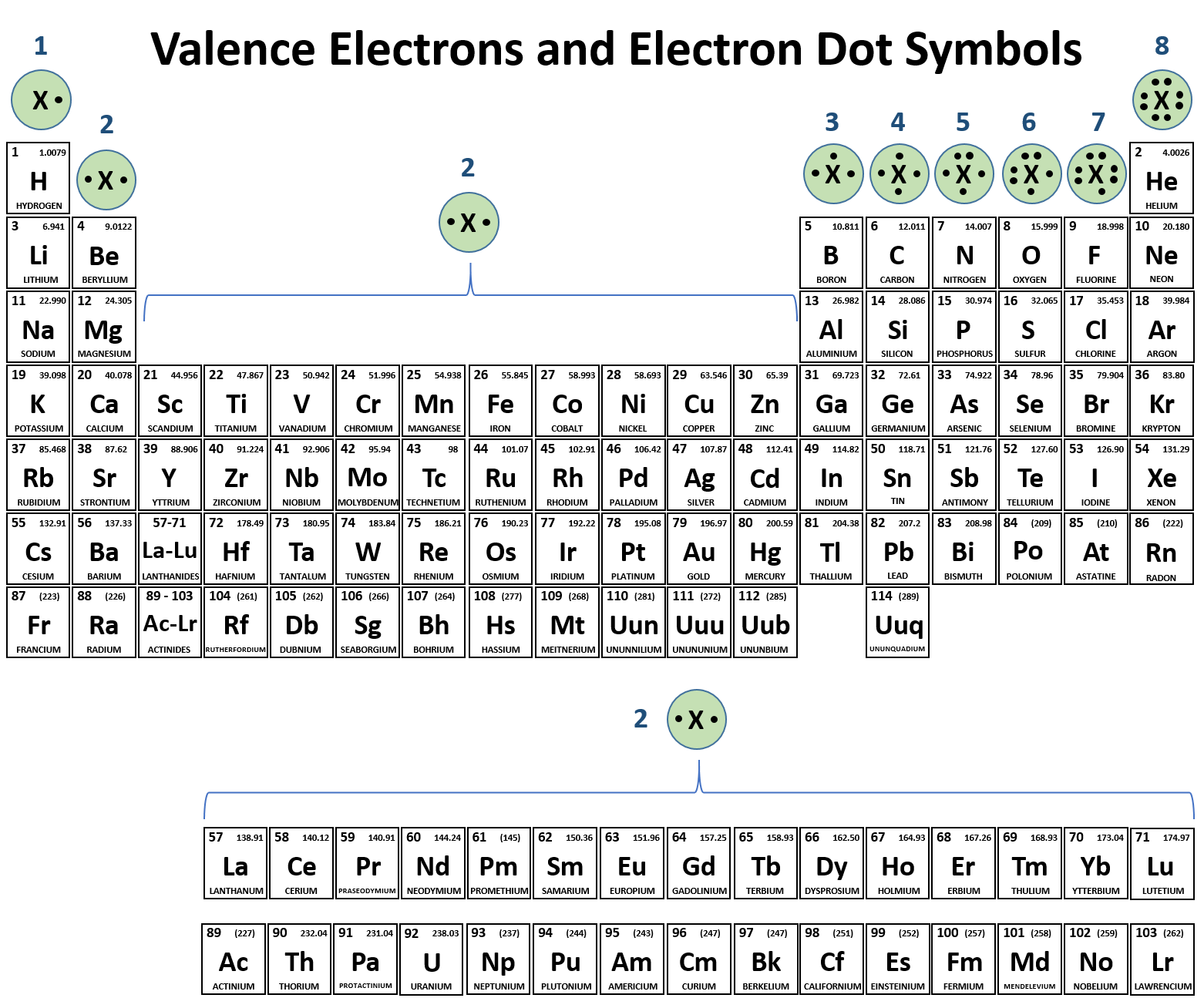
How To Know How Many Bonds An Element Can Form - Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since. Consider as an example an atom of sodium,. Web there is a quick way to work out how many covalent bonds an element will form. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. Web how can you tell how many bonds an atom can form? The table below shows the number of bonds formed by elements in groups. This is summarized in the table below. The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. Web best answer copy by which group (or column) it's in.
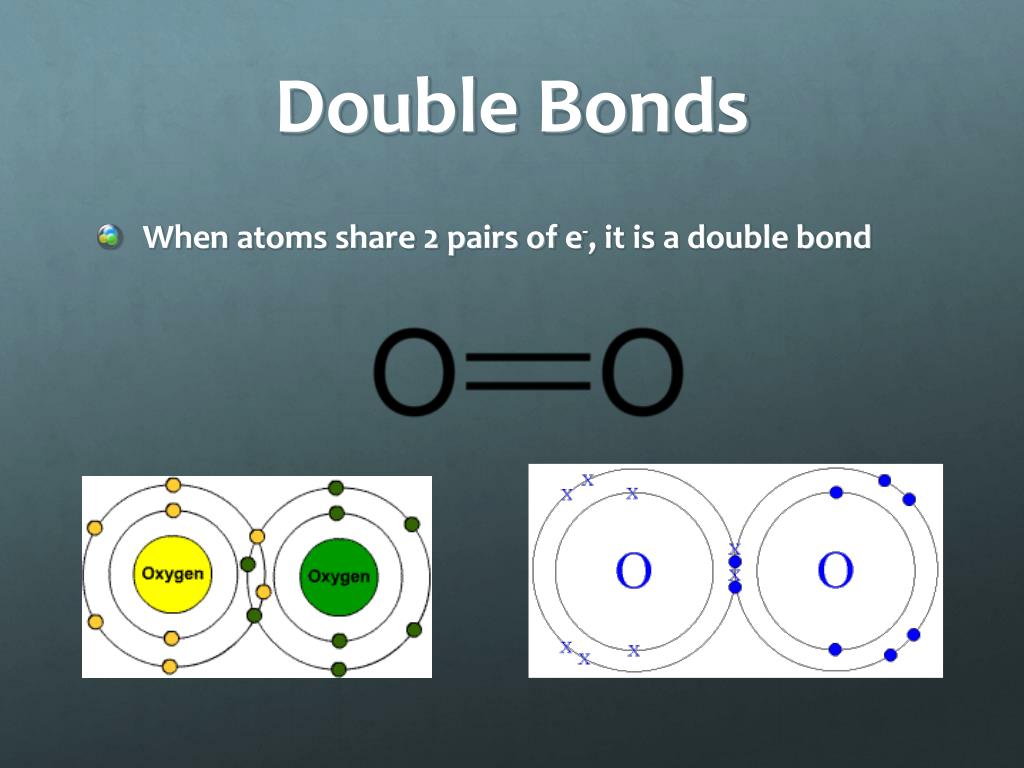
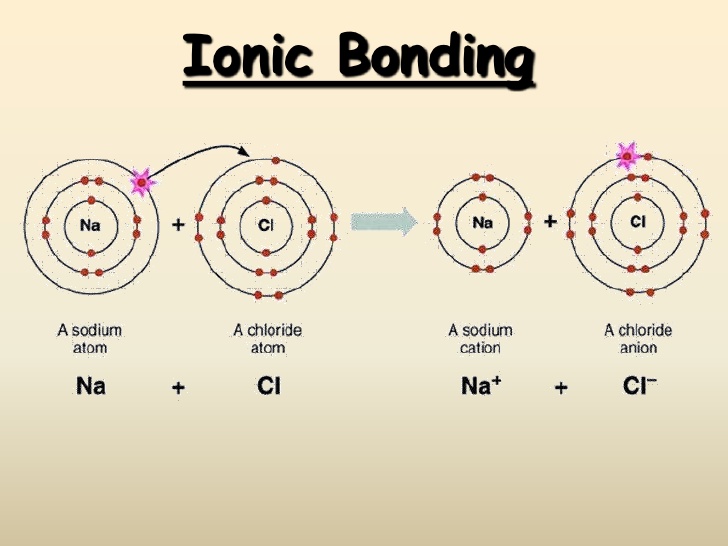
It's named a covalent bond. Web there are three basic ways that the outer electrons of atoms can form bonds: Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since. The first way gives rise to what is called an ionic bond. Web for most elements, a full outer shell is eight electrons. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. For example, in methane (ch 4 _4 4 start subscript, 4, end subscript), carbon forms covalent bonds with. The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. Web carbon atoms may thus form bonds to as many as four other atoms. The number of covalent bonds is equal to eight minus the group number.
The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. For example, in methane (ch 4 _4 4 start subscript, 4, end subscript), carbon forms covalent bonds with. Web there are three basic ways that the outer electrons of atoms can form bonds: Web a covalent bond is formed when two atoms share electron pairs. Web for most elements, a full outer shell is eight electrons. 4 to 7 (iupac groups 14 to 17). Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. Web there is a quick way to work out how many covalent bonds an element will form. Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since. The first way gives rise to what is called an ionic bond.
Single, Double, and Triple Bonds
It's named a covalent bond. The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. Web for most.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Web carbon atoms may thus form bonds to as many as four other atoms. 4 to 7 (iupac groups 14 to 17). Web there are three basic ways that the outer electrons of atoms can form bonds: It's named a covalent bond. The number of covalent bonds is equal to eight minus the group number.
Covalent Bonds Biology for NonMajors I
This is summarized in the table below. The columns of the periodic table, which contain elements that show a family resemblance, are called. It's named a covalent bond. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. The first way gives rise to what is called an ionic bond.
11 Types of scientific changes with examples
It's named a covalent bond. The number of covalent bonds is equal to eight minus the group number. Web how can you tell how many bonds an atom can form? In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two. Web best answer copy by which group (or column) it's in.
PPT Chapter 22 Chemical Bonding PowerPoint Presentation, free
The columns of the periodic table, which contain elements that show a family resemblance, are called. The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. Web a covalent bond is formed when two atoms share electron pairs. Web best answer copy by which.
How to Predict number of bonds each element forms ChemSimplified
For example, in methane (ch 4 _4 4 start subscript, 4, end subscript), carbon forms covalent bonds with. Web how can you tell how many bonds an atom can form? This is summarized in the table below. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight.
How to Predict number of bonds each element forms ChemSimplified
In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two. The first way gives rise to what is called an ionic bond. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. It's named a.
Elements and Chemical Bonds
The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. Web best answer copy by which group (or column) it's in. Web a covalent bond is formed when two atoms share electron pairs. Web the number of covalent bonds that an atom can form.
Elements and Chemical Bonds
Web how can you tell how many bonds an atom can form? Web carbon atoms may thus form bonds to as many as four other atoms. The first way gives rise to what is called an ionic bond. Web a covalent bond is formed when two atoms share electron pairs. 4 to 7 (iupac groups 14 to 17).
What are similarities of covalent and ionic bonding? Socratic
Web there are three basic ways that the outer electrons of atoms can form bonds: This is summarized in the table below. Web carbon atoms may thus form bonds to as many as four other atoms. Web there is a quick way to work out how many covalent bonds an element will form. Web the total number of electrons around.
The Amount Of Hydrogen Atoms That Can Be Bond (Or Any Other Atom) Can Be Calculated Most Of The Time Using The Octet Rule, That States.
Consider as an example an atom of sodium,. Web the number of covalent bonds that an atom can form depends on the number of available electrons found in its outermost (valence) shell. Web a covalent bond is formed when two atoms share electron pairs. This is summarized in the table below.
The Columns Of The Periodic Table, Which Contain Elements That Show A Family Resemblance, Are Called.
The single place digit refers to the number of electrons in the valence shell of the elements in that group, with. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two. The table below shows the number of bonds formed by elements in groups.
Web Best Answer Copy By Which Group (Or Column) It's In.
The number of covalent bonds is equal to eight minus the group number. 4 to 7 (iupac groups 14 to 17). Web for most elements, a full outer shell is eight electrons. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
Web How Can You Tell How Many Bonds An Atom Can Form?
The first way gives rise to what is called an ionic bond. Web carbon atoms may thus form bonds to as many as four other atoms. Web there are three basic ways that the outer electrons of atoms can form bonds: It's named a covalent bond.