Hydrogen And Nitrogen Combine To Form Ammonia
Hydrogen And Nitrogen Combine To Form Ammonia - Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web 1 day agofor the second quarter of 2023, cvr partners’ average realized gate prices for uan showed a reduction over the prior year, down 43 percent to $316 per ton, and. Write a balanced chemical equation for this reaction? Web hydrogen and nitrogen combine to form ammonia. Combination reaction explanation:hydrogen (h2) combines with nitrogen (n2) to form a single compound, ammonia (nh3) in a combination reaction. Web hydrogen and nitrogen combine in a synthesis reaction to form ammonia (nh 3) in the haber process. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web n2+3h2→2nh3 [relative atomic masses of n=14,h=1 ]. Ammonia is usually produces when hydrogen. I mean to say that, when hydrogen (g) and nitrogen (g) combines together.
Web nitrogen and hydrogen combine to form ammonia via the following reaction: When nitrogen and hydrogen bond, nitrogen pulls the electrons from hydrogen toward itself. The reaction can be written as: Hydrogen reacts with nitrogen to produce ammonia. Web hydrogen gas combines with nitrogen to form ammonia. Web answer answered 1. Write a balanced chemical equation for this reaction? I mean to say that, when hydrogen (g) and nitrogen (g) combines together. Web nitrogen and hydrogen combine together to form ammonia. Representation of the chemical reaction:
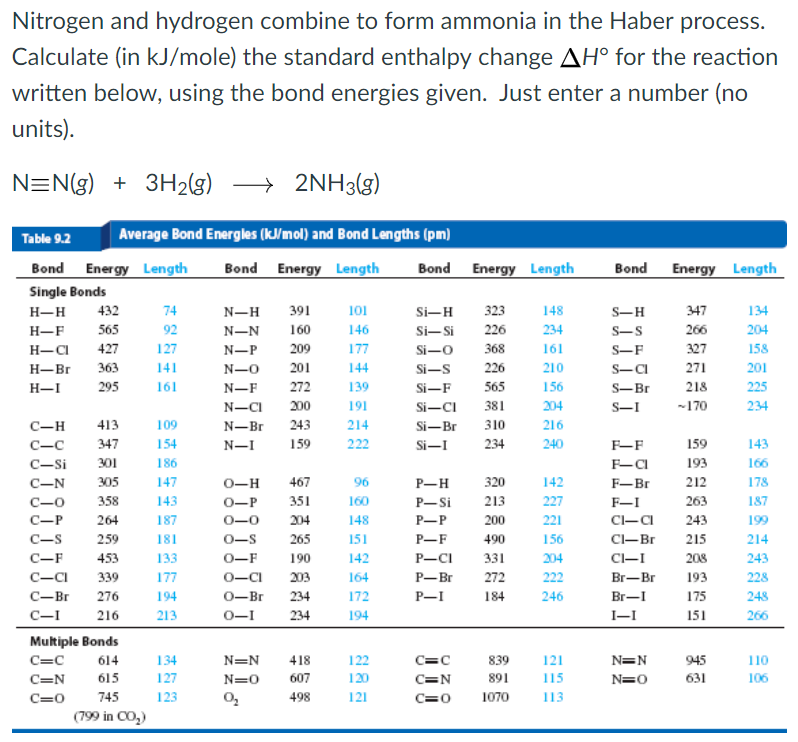
Translate the following into a chemical equation and balance the equation. Web nitrogen and hydrogen combine to form ammonia in the haber process. Nitrogen and hydrogen combine to form ammonia in the haber process. How many moles of ammonia will be produced using 2.0 moles of. The mass of nitrogen and hydrogen which combine together to form 6.8 g ammonia is. I mean to say that, when hydrogen (g) and nitrogen (g) combines together. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Using bond energy data, what is ah®ren for the. Representation of the chemical reaction: Hydrogen reacts with nitrogen to produce ammonia.
Solved Under certain conditions, the substances bromine and
Web nitrogen and hydrogen combine to form ammonia via the following reaction: Nitrogen and hydrogen combine to form ammonia in the haber process. Translate the following into a chemical equation and balance the equation. Web hydrogen gas combines with nitrogen to form ammonia. Web answer answered 1.
Solved Nitrogen and hydrogen combine to form ammonia in the
Calculate (in kj) the standard enthalpy change h for the reaction written below, using the bond energies. When nitrogen and hydrogen bond, nitrogen pulls the electrons from hydrogen toward. Web nitrogen gas and hydrogen gas combine to produce ammonia gas. Write a balanced chemical equation for this reaction? 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass.
Under certain conditions, the substances nitrogen and hydrogen combine
Write a balanced chemical equation for this reaction? Transport of the reactants from the gas phase through the boundary layer to the surface of the catalyst. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; I mean to say that, when hydrogen (g) and nitrogen (g) combines together..
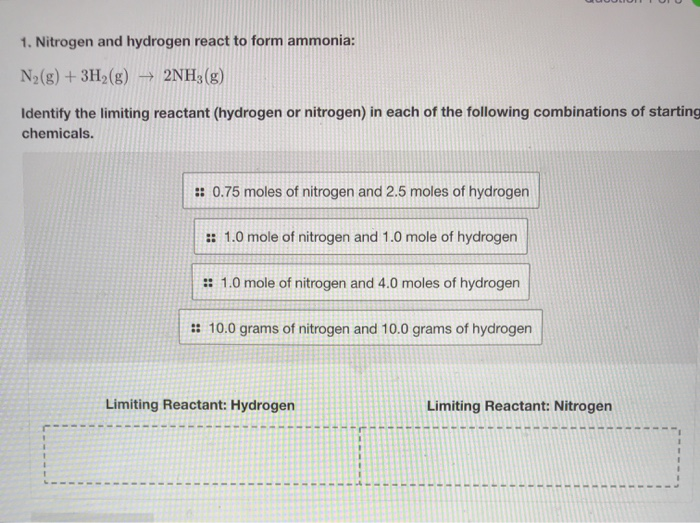
Solved 1. Nitrogen and hydrogen react to form ammonia N2(g)
Calculate (in kj) the standard enthalpy change ah for the reaction written below, using. Hydrogen reacts with nitrogen to produce ammonia. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web 1 day agofor the second quarter of 2023, cvr partners’ average realized gate prices for uan showed a reduction over the prior year, down.
Nitrogen gas and hydrogen gas combine to form gaseous ammonia
Hydrogen reacts with nitrogen to produce ammonia. Web nitrogen gas and hydrogen gas combine to produce ammonia gas. The reaction can be written as: Nitrogen and hydrogen combine to form ammonia in the haber process. Combination reaction explanation:hydrogen (h2) combines with nitrogen (n2) to form a single compound, ammonia (nh3) in a combination reaction.
Solved 10 g of nitrogen is reacted with 5.0 g of hydrogen to
Web nitrogen and hydrogen combine to form ammonia via the following reaction: Web hydrogen and nitrogen combine in a synthesis reaction to form ammonia (nh 3) in the haber process. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; N2 + 3h2→ 2nh3 [relative atomic masses of n.
Is Ammonia an Acid or Base? » Science ABC
Web n2+3h2→2nh3 [relative atomic masses of n=14,h=1 ]. Calculate (in kj) the standard enthalpy change ah for the reaction written below, using. Web hydrogen and nitrogen combine to form ammonia. Web answer answered 1. Web nitrogen and hydrogen combine to form ammonia via the following reaction:
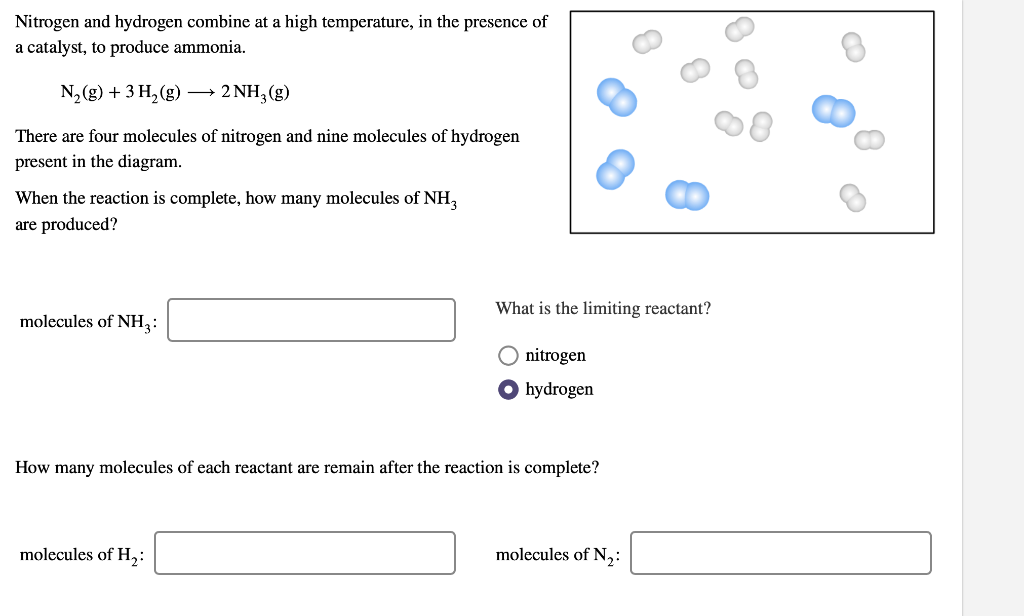
Solved Nitrogen and hydrogen combine at a high temperature,
Web hydrogen gas combines with nitrogen to form ammonia. Transport of the reactants from the gas phase through the boundary layer to the surface of the catalyst. Nitrogen and hydrogen combine to form ammonia in the haber process. Web 1 day agofor the second quarter of 2023, cvr partners’ average realized gate prices for uan showed a reduction over the.
Solved Nitrogen And Hydrogen Combine To Form Ammonia In T...
Web nitrogen and hydrogen combine to form ammonia in the haber process. Hydrogen and nitrogen combine to form ammonia. If 56.4 g of nitrogen are reacted completely with plenty of hydrogen, what. When nitrogen and hydrogen bond, nitrogen pulls the electrons from hydrogen toward. Combination reaction explanation:hydrogen (h2) combines with nitrogen (n2) to form a single compound, ammonia (nh3) in.
Solved Nitrogen and hydrogen react to produce ammonia
Nitrogen and hydrogen combine to form ammonia in the haber process. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web nitrogen gas and hydrogen gas combine to form ammonia (nh3) gas. How many moles of ammonia will be produced using 2.0 moles of. Web nitrogen and hydrogen combine together to form ammonia.
H 2 Hydrogen + N 2 Nittrogen → Nh 3.
1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. The mechanism of ammonia synthesis contains the following seven elementary steps: I mean to say that, when hydrogen (g) and nitrogen (g) combines together. N2 + 3h2→ 2nh3 [relative atomic masses of n = 14 u, h = 1 u] the mass of nitrogen and.
H 2 ( G) Hydrogen + N 2 ( G) Nitrogen ⇌ Nh 3 ( G).
Nitrogen and hydrogen combine to form ammonia in the haber process. The mass of nitrogen and hydrogen which combine together to form 6.8 g ammonia is. Web n2+3h2→2nh3 [relative atomic masses of n=14,h=1 ]. Web nitrogen and hydrogen combine to form ammonia via the following reaction:
If 56.4 G Of Nitrogen Are Reacted Completely With Plenty Of Hydrogen, What.
Calculate (in kj) the standard enthalpy change h for the reaction written below, using the bond energies. When nitrogen and hydrogen bond, nitrogen pulls the electrons from hydrogen toward. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web answer answered 1.
Calculate (In Kj) The Standard Enthalpy Change Ah For The Reaction Written Below, Using.
Hydrogen reacts with nitrogen to produce ammonia. Nitrogen and hydrogen combine to form ammonia in the haber process. Web nitrogen and hydrogen combine to form ammonia which is the only product derived from this reaction. Using bond energy data, what is ah®ren for the.