Hydrogen Atom Drawing
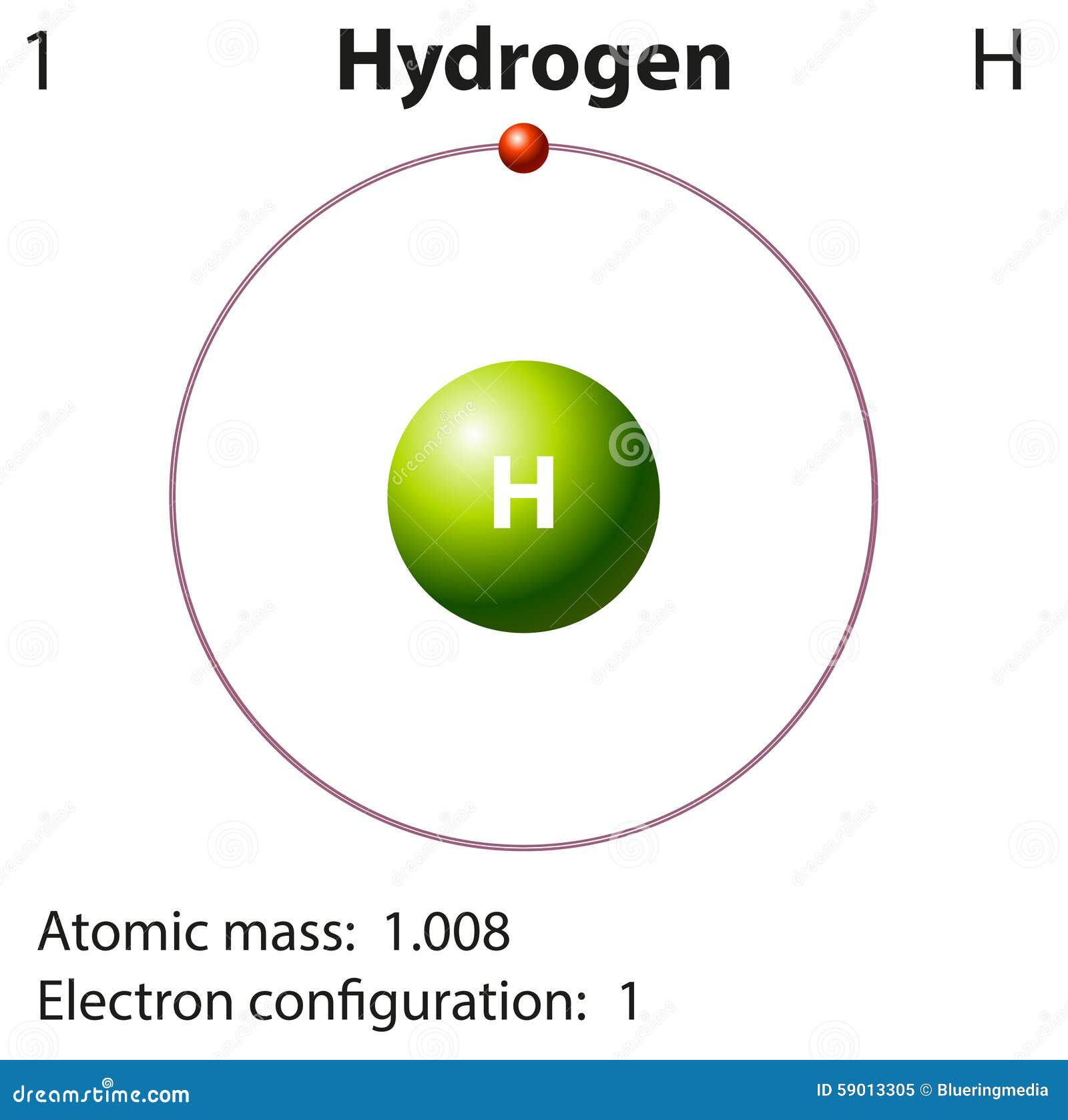
Hydrogen Atom Drawing - Quantum numbers for the first four shells. For \(e \geq 0\), the energy is a continuum, since the electron is in fact a free particle. In bohr’s model, the electron is pulled around the proton in a perfectly circular orbit by an attractive coulomb force. A picture of a hydrogen atom can be found here. The continuum represents states of an electron and proton in. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web choose from hydrogen atom stock illustrations from istock. The electronegativity value for carbon (c) and hydrogen (h) is 2.55 and 2.1 respectively, so the difference in their electronegativity values is only 0.45 (<0.5 criteria); In 1913, after returning to copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on.
Web it has the lowest density of all gases. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. For \(e \geq 0\), the energy is a continuum, since the electron is in fact a free particle. Web equation \(\ref{1.8.21}\) gives the energies of the electronic states of the hydrogen atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. A picture of a hydrogen atom can be found here. The second shell has 2 subshells: A single electron (white) occupies the first of successive electron. We’ll use a bohr diagram to visually represent where the electrons. This means that the first shell can hold 2 electrons.
Structural formula, balls and sticks model, hydrogen bonding. Web every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). Bohr's model does not work for systems with more than one electron. Also, helium is shown in group 8a, but it only has two valence electrons. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. The electronegativity value for carbon (c) and hydrogen (h) is 2.55 and 2.1 respectively, so the difference in their electronegativity values is only 0.45 (<0.5 criteria); Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. 1), the most common isotope of the element hydrogen. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Depiction of a hydrogen atom showing the diameter as about twice the bohr model radius.
Chemistry model of molecule hydrogen H2 scientific element. Integrated
Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. The electronegativity value for carbon (c) and hydrogen (h) is 2.55 and 2.1 respectively, so the difference in their electronegativity values is only 0.45 (<0.5 criteria); Precisely determined thermonuclear rates of these reactions lie at the foundation of the.
Hydrogen Atom Water Molecule Molecular Orbital Diagram, PNG
The hydrogen atom is then left with a. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. The continuum represents states of an electron and proton in. 1 that’s not including.
How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
Web it has the lowest density of all gases. Web a very simple drawing of an hydrogen atom with a nucleus and an electron :) Web the ptco/mxene catalyst exhibits a superior her activity with a low overpotential of 60 and 152 mv at current densities of −10 and −100 ma/cm 2, respectively, and excellent working durability. Electrons in the.
Diagram Representation of the Element Hydrogen Stock Vector
Web the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. A picture of a hydrogen atom can be found here. 1), the most common isotope of the element hydrogen. In 1913, after returning to copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on. Vector.
A hydrogen atom with an electron chemical model Vector Image
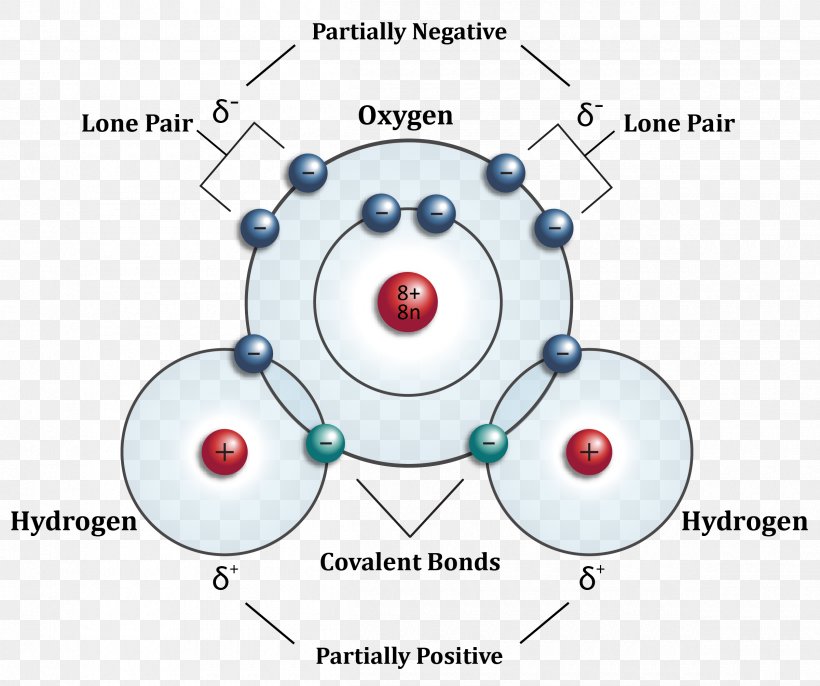
Electrons in the same subshell have the same energy, while electrons in different shells or subshells have. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. In bohr’s model, the electron is pulled around the proton in a perfectly circular orbit by an attractive coulomb force. Web figure 7.
Hydrogen Atom Diagram Concept Stock Vector Illustration of abstract
The electronegativity value for carbon (c) and hydrogen (h) is 2.55 and 2.1 respectively, so the difference in their electronegativity values is only 0.45 (<0.5 criteria); (image not to scale) a hydrogen atom is an atom of the chemical element hydrogen. E ( n) = − 1 n 2 ⋅ 13.6 ev. Web figure 7 shows an energy level diagram.
Hydrogen atom diagram concept Royalty Free Vector Image
The hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton ().in bohr’s model, the electron is pulled around the proton in a perfectly circular orbit by an attractive coulomb. All of the electron pairs—shared and unshared—repel each other. Structural formula, balls and sticks model, hydrogen bonding. A single electron (white) occupies the first.
Diagram representation element hydrogen Royalty Free Vector
Bohr's model does not work for systems with more than one electron. In 1913, after returning to copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Web the ptco/mxene catalyst exhibits a superior her activity.
Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
Web in this video we'll look at the atomic structure and bohr model for the hydrogen atom (h). Web it has the lowest density of all gases. Web the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. A picture of a hydrogen atom can be found here. Bohr's.
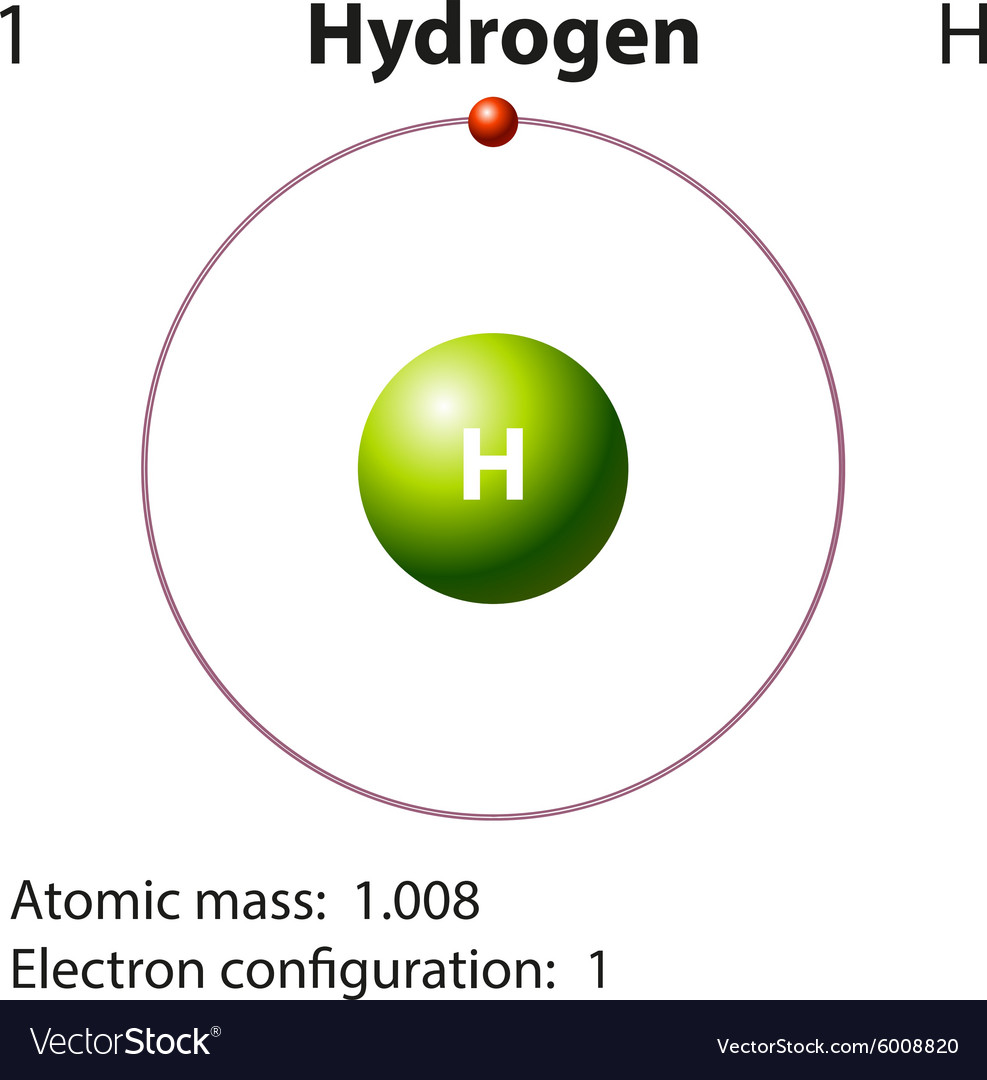
Symbol and electron diagram for hydrogen Vector Image
We’ll use a bohr diagram to visually represent where the electrons. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web how did scientists figure out the structure of atoms without looking at them? The continuum represents states of an electron and proton in. The quantum mechanical model.
Web It Has The Lowest Density Of All Gases.
The continuum represents states of an electron and proton in. Web a very simple drawing of an hydrogen atom with a nucleus and an electron :) Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Quantum numbers for the first four shells.
Its Nucleus Consists Of A Single Proton (Red) And No Neutrons.
E ( n) = − 1 n 2 ⋅ 13.6 ev. Also, helium is shown in group 8a, but it only has two valence electrons. 1 that’s not including “dark matter,” which is beyond the scope. The electrons are thus equally.
In 1913, After Returning To Copenhagen, He Began Publishing His Theory Of The Simplest Atom, Hydrogen, Based On.
Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web choose from hydrogen atom stock illustrations from istock. A picture of a hydrogen atom can be found here.
Bohr Became Convinced Of Its Validity And Spent Part Of 1912 At Rutherford’s Laboratory.
The hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton ().in bohr’s model, the electron is pulled around the proton in a perfectly circular orbit by an attractive coulomb. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. Web the ptco/mxene catalyst exhibits a superior her activity with a low overpotential of 60 and 152 mv at current densities of −10 and −100 ma/cm 2, respectively, and excellent working durability.