Hydrogen Bohr Model Drawing
Hydrogen Bohr Model Drawing - Web bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. In this video we'll look at the atomic structure and bohr model for the hydrogen atom (h). Want to join the conversation? Create the nucleus, and then proceed to sketch the first electron shell. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. Web using equation for bohr model radii to draw shell model for n=1 to 3, and calculating the velocity of a ground state electron. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Web bohr's model of the hydrogen atom was the first to incorporate quantum theory, and the key idea of his model was that electrons occupy discrete orbitals. Want to join the conversation? Bohr's model was only successful in calculating energy levels for the hydrogen atom.
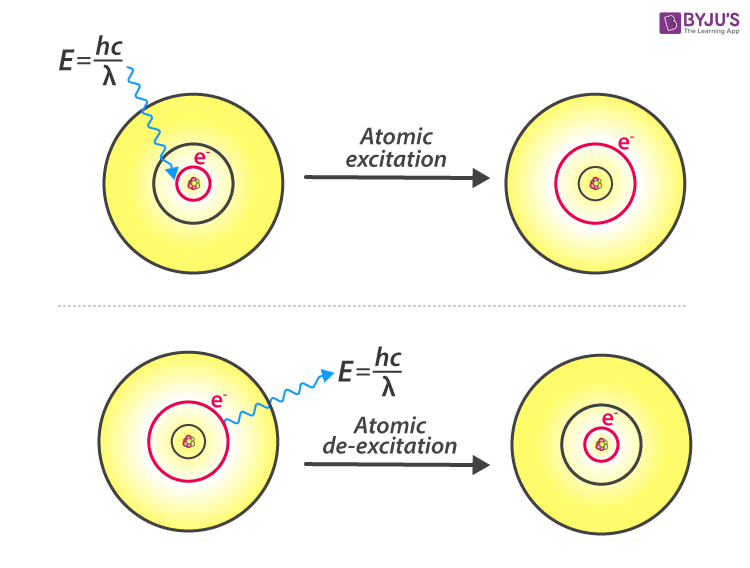
Web using equation for bohr model radii to draw shell model for n=1 to 3, and calculating the velocity of a ground state electron. Bohr's original attempt to extend his model to molecules [2] has long been regarded as a complete failure. They also help us explain and predict the behavior of atoms. Web how did scientists figure out the structure of atoms without looking at them? So, we represent atoms using models. Web the bohr model is used to describe the structure of hydrogen energy levels. Web bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Models help us visualize atomic structure. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Use the rydberg equation to calculate energies of light emitted or absorbed by hydrogen atoms.
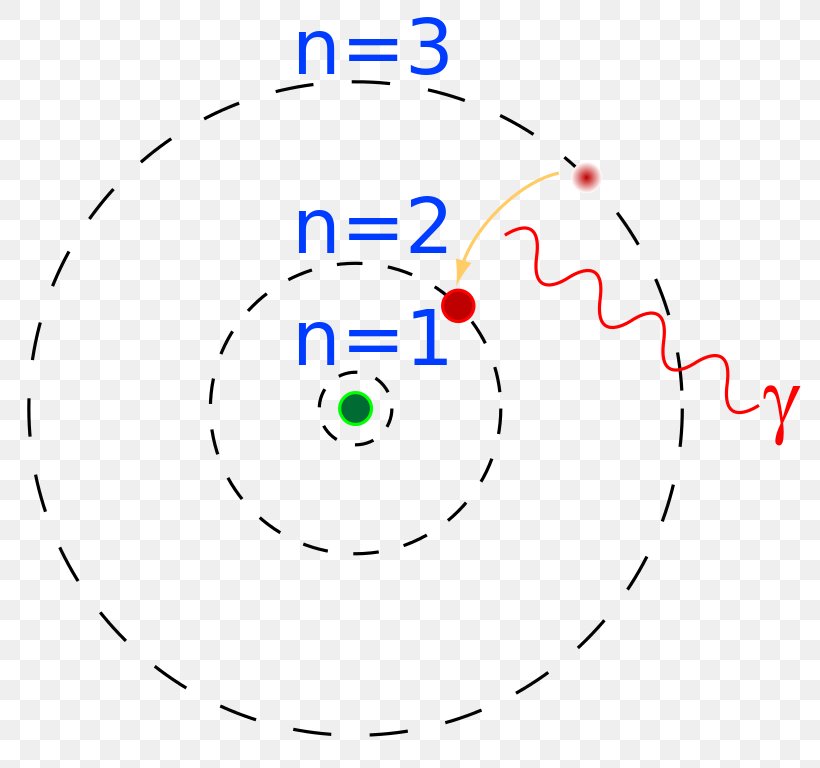
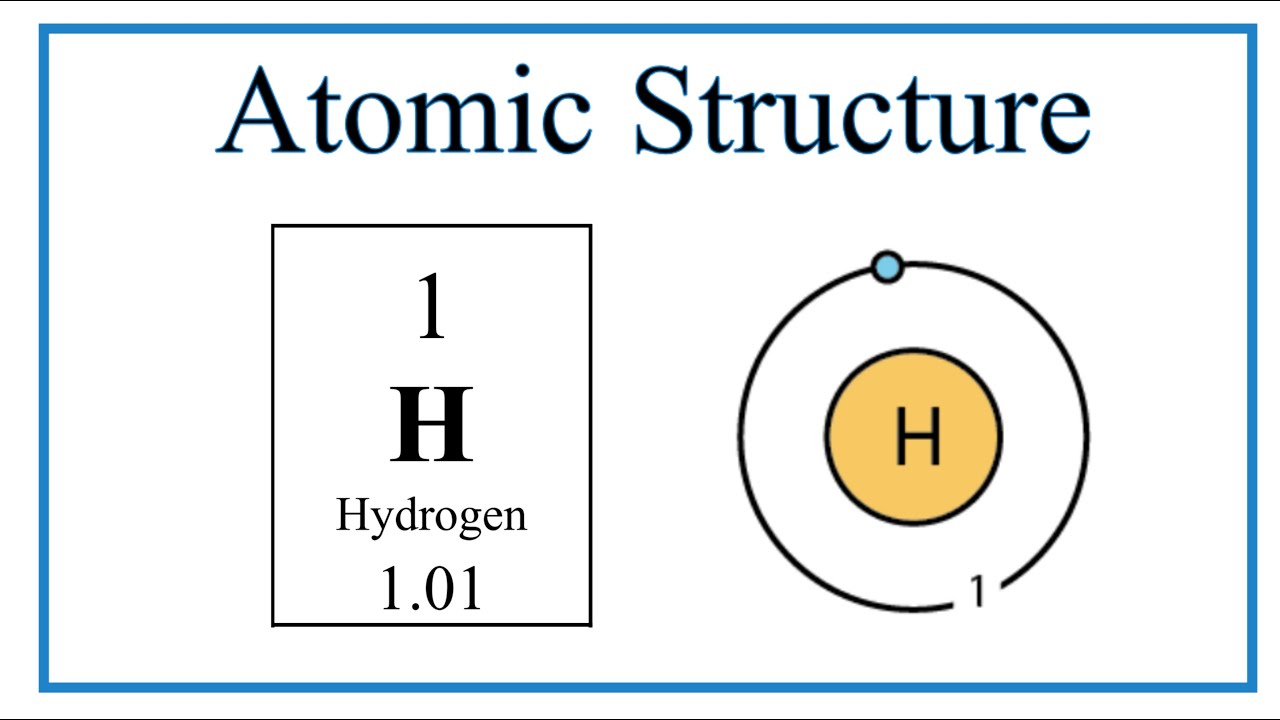
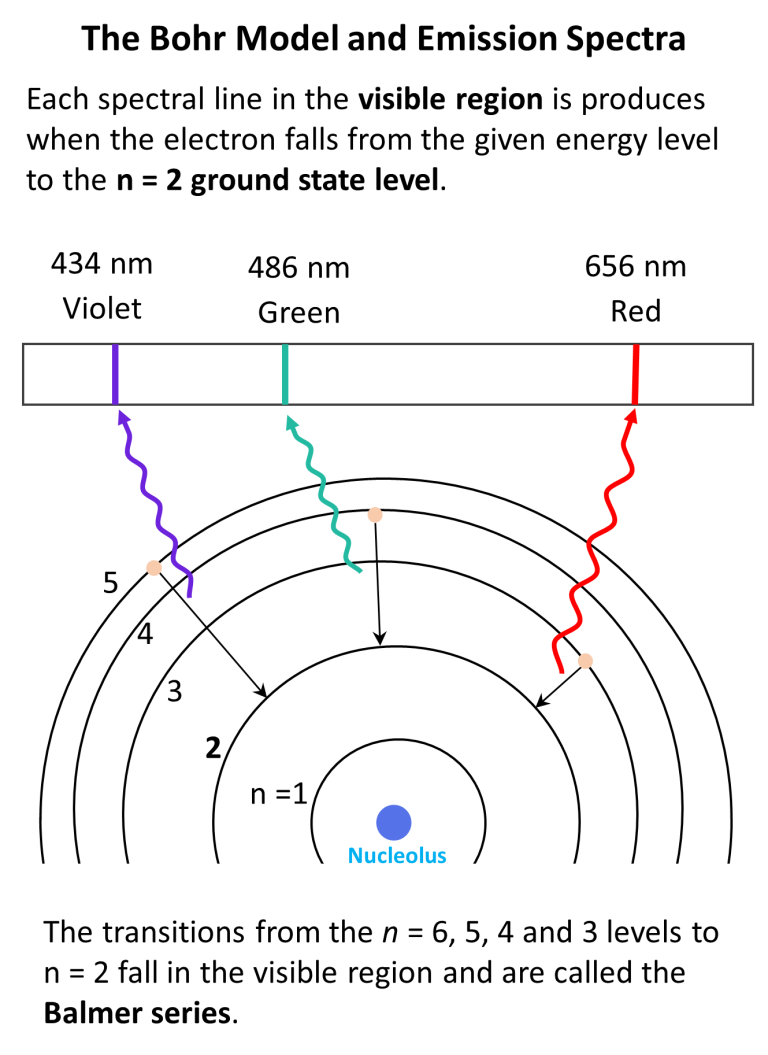
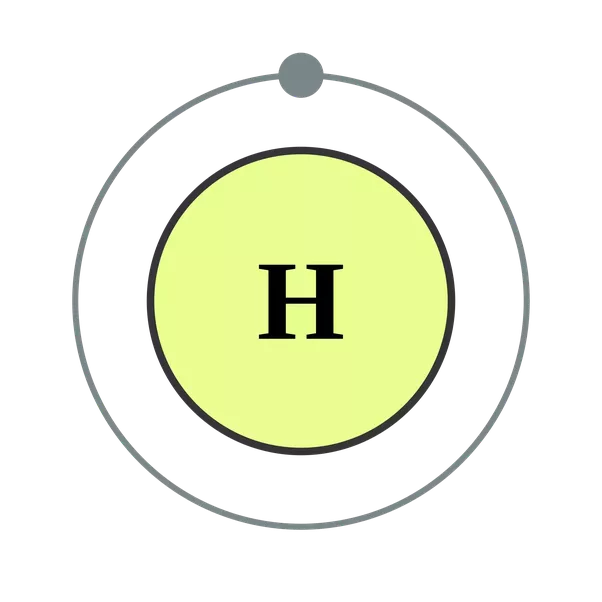
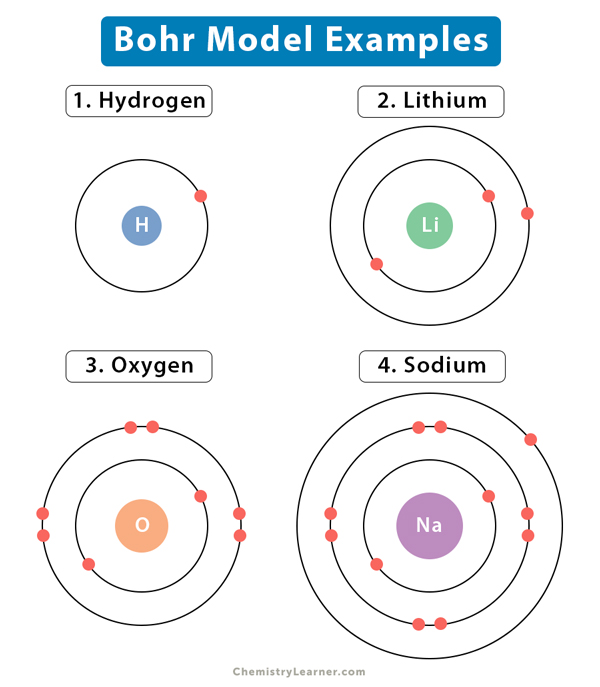
The image below represents shell structure, where each shell is associated with principal quantum number n. The bohr model for the hydrogen atom marks the historic paradigm shift in the development of the quantum theory of matter [1]. Web to draw the hydrogen bohr model, begin by noting the 1 proton and 0 neutrons. Calculating electron energy for levels n=1 to 3. It is observed that excited hydrogen atoms emit light at. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. Web bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Atomic structure (bohr model) for hydrogen (h) watch on. Web the existence of the atomic spectra is support for bohr's model of the atom.
Bohr Model Hydrogen Atom Diagram
Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. Web bohr's model of the hydrogen atom was the first to incorporate quantum theory, and the key idea of his model was that electrons occupy discrete orbitals. Bohr's original attempt to extend his model to molecules [2] has.
Atomic Structure (Bohr Model) for Hydrogen (H) YouTube
So, we represent atoms using models. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. Web using equation for bohr model radii to draw shell model for n=1 to 3, and calculating.
Bohr Atomic Model Of Hydrogen
Web using equation for bohr model radii to draw shell model for n=1 to 3, and calculating the velocity of a ground state electron. Web bohr's model of the hydrogen atom was the first to incorporate quantum theory, and the key idea of his model was that electrons occupy discrete orbitals. Web the bohr model is used to describe the.
Bohr Model of the Hydrogen Atom Chemistry Steps
The bohr model for the hydrogen atom marks the historic paradigm shift in the development of the quantum theory of matter [1]. Historically, bohr’s model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. Web the bohr model is used to describe the structure of hydrogen energy levels..
Explain the Bohr model of the hydrogen atom
Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Web describe the bohr model of the hydrogen atom. So, we represent atoms using models. Web bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. The image below represents.
Bohr model scientific hydrogen atom Royalty Free Vector
Bohr model of the hydrogen atom. Atoms are way too small to see with the naked eye (and even most microscopes). Web bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Learn how bohr models are used to represent atoms. The following are his key.
1.8 The Bohr Theory of the Hydrogen Atom Chemistry LibreTexts
Web the existence of the atomic spectra is support for bohr's model of the atom. Web bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. A full valence shell is the most stable electron configuration. Web to draw the hydrogen bohr model, begin by noting the 1 proton and 0 neutrons. They also help.
Describe Bohr’s model of the hydrogen atom. bitWise Academy
Atoms are way too small to see with the naked eye (and even most microscopes). Web bohr’s theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics. Try out different models by shooting light at the atom. Web bohr's model of the hydrogen atom was the first to incorporate quantum theory, and the.
Bohr Atomic Model Of Hydrogen
Write protons, neutrons, and electrons of hydrogen atom. Create the nucleus, and then proceed to sketch the first electron shell. Want to join the conversation? So, we represent atoms using models. Atomic structure (bohr model) for hydrogen (h) watch on.
Hydrogen Atom Hydrogen Bohr Diagram
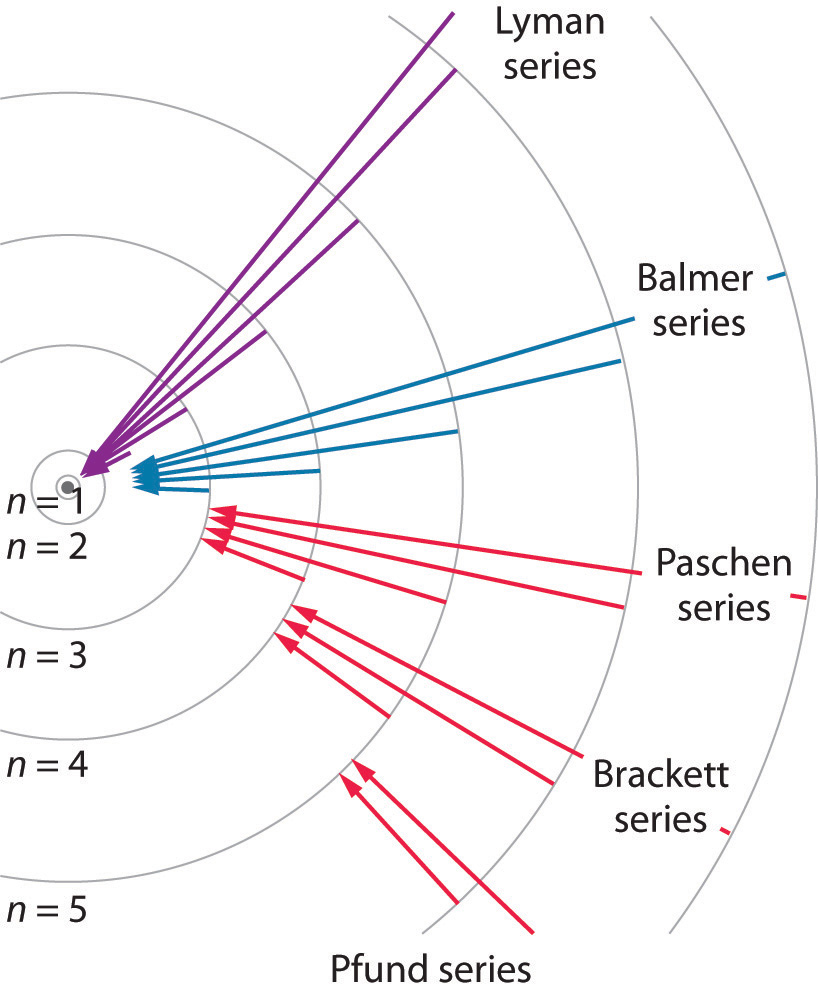
Calculating electron energy for levels n=1 to 3. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Try out different models by shooting light at the atom. Unfortunately, bohr could not explain why the electron should be restricted to particular orbits. Learn how bohr models are used.
Historically, Bohr’s Model Of The Hydrogen Atom Is The Very First Model Of Atomic Structure That Correctly Explained The Radiation Spectra Of Atomic Hydrogen.
Calculating electron energy for levels n=1 to 3. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web how did scientists figure out the structure of atoms without looking at them? Web bohr’s theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics.
Create The Nucleus, And Then Proceed To Sketch The First Electron Shell.
Web the existence of the atomic spectra is support for bohr's model of the atom. 14k views 1 year ago. Web describe the bohr model of the hydrogen atom. Web summarize how bohr’s quantum model of the hydrogen atom explains the radiation spectrum of atomic hydrogen.
In This Video We'll Look At The Atomic Structure And Bohr Model For The Hydrogen Atom (H).
Web bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. A visualization of the bohr model and the hydrogen spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Web bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the danish physicist niels bohr.
Is This Equation Applicable Only Hydrogen Atom?
Use the rydberg equation to calculate energies of light emitted or absorbed by hydrogen atoms. Web using equation for bohr model radii to draw shell model for n=1 to 3, and calculating the velocity of a ground state electron. Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. A full valence shell is the most stable electron configuration.


.PNG)