Ionic Bonds Form Between Atoms With Complementary
Ionic Bonds Form Between Atoms With Complementary - These ions attract each other. Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron configurations describe the formation of covalent bonds define electronegativity and assess the polarity of covalent bonds Ions are created when an atom loses or gains an electron. Ionic bonds result from the attraction between oppositely charged ions. Web types of chemical bonds including covalent, ionic, and hydrogen bonds and london dispersion forces. Electron transfer produces negative ions called anions and positive ions called cations. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ionic bonds require at least one electron donor and one electron acceptor. Web atoms interact with each other through the formation of chemical bonds. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei.
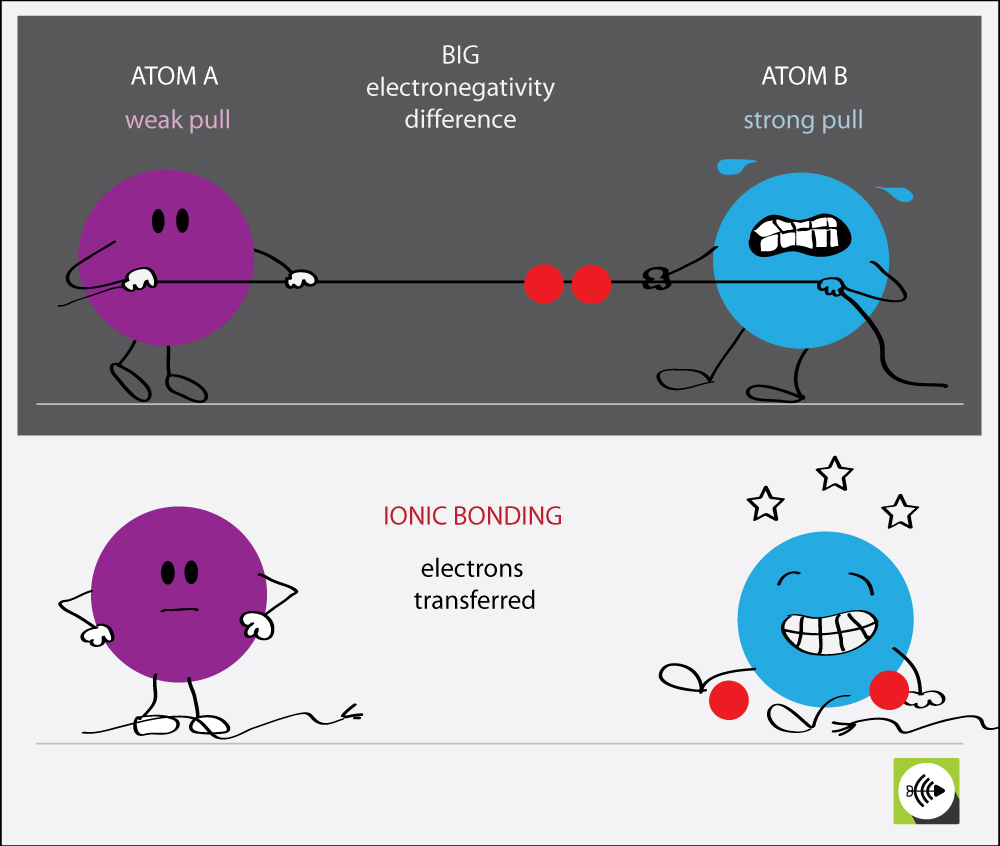
Web atoms interact with each other through the formation of chemical bonds. Web in ionic bonding, atoms transfer electrons to each other. Ions are created when an atom loses or gains an electron. These ions attract each other. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. One type of chemical bond is an ionic bond. Ionic bonds result from the attraction between oppositely charged ions. Instead, they’re usually interacting with other atoms (or groups of atoms). For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. Let’s examine the ionic bond in sodium chloride.
Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. One type of chemical bond is an ionic bond. An example of a covalent compound is ammonia. Web atoms interact with each other through the formation of chemical bonds. Ionic bonds require at least one electron donor and one electron acceptor. In ionic bonding, electrons are considered to be transferred completely from one atom to another atom (or group of atoms), forming ions of opposite charge. Ions are created when an atom loses or gains an electron. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. Instead, they’re usually interacting with other atoms (or groups of atoms).
Chapter 2 Atoms, Molecules and Life Chemistry)
Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. One type of chemical bond is an ionic bond. Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron configurations describe the formation.
Ionic Bonding Presentation Chemistry
Web in ionic bonding, atoms transfer electrons to each other. Ionic bonds result from the attraction between oppositely charged ions. Instead, they’re usually interacting with other atoms (or groups of atoms). Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron.
이온 성 공유 결합과 금속 결합의 차이점 2021 뉴스
Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. Web types of chemical bonds including covalent, ionic, and hydrogen bonds.
Ionic Bond Definition, Types, Properties & Examples
Introduction living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table. Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the.
Chemical Bonds
These ions then attract each other electrostatically to form a stable crystalline lattice. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web compounds can be covalent or ionic. Web glossary summary glossary.
Electronegativity Bond Scale Surfguppy Chemistry made easy for
Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron configurations describe the formation of covalent bonds define electronegativity and assess the polarity of covalent bonds Ionic bonds require at least one electron donor and one electron acceptor. Such a bond.
Ionic Properties
In ionic bonding, electrons are considered to be transferred completely from one atom to another atom (or group of atoms), forming ions of opposite charge. An example of a covalent compound is ammonia. Ionic bonds result from the attraction between oppositely charged ions. Ions are created when an atom loses or gains an electron. Web compounds can be covalent or.
Examples of Ionic Bonds and Ionic Compounds
Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. These ions then attract each other electrostatically to form a stable crystalline lattice. Web in ionic bonding, atoms transfer electrons to each other. In ionic bonding, electrons are considered to be transferred completely from one atom to another atom (or group of.
Student Exploration Ionic Bonds Answer Key Quizlet / Ionic Bonds Gizmo
These ions then attract each other electrostatically to form a stable crystalline lattice. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. One type of chemical bond is an ionic bond. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in.
Ionic Bond Definition, Types, Properties & Examples
Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron configurations describe the formation of covalent bonds define electronegativity and assess the polarity of covalent bonds Web ionic bonding is the complete transfer of valence electron (s) between atoms and is.
Introduction Living Things Are Made Up Of Atoms, But In Most Cases, Those Atoms Aren’t Just Floating Around Individually.
Ionic bonds result from the attraction between oppositely charged ions. Web types of chemical bonds including covalent, ionic, and hydrogen bonds and london dispersion forces. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. In ionic bonding, electrons are considered to be transferred completely from one atom to another atom (or group of atoms), forming ions of opposite charge.
These Ions Attract Each Other.
Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An example of a covalent compound is ammonia. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Web in ionic bonding, atoms transfer electrons to each other.
For Example, Sodium Cations (Positively Charged Ions) And Chlorine Anions (Negatively Charged Ions) Are Connected Via Ionic Bonds In Sodium Chloride, Or Table.
Let’s examine the ionic bond in sodium chloride. Ions are created when an atom loses or gains an electron. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. These ions then attract each other electrostatically to form a stable crystalline lattice.
Ionic Bonds Require At Least One Electron Donor And One Electron Acceptor.
Electron transfer produces negative ions called anions and positive ions called cations. One type of chemical bond is an ionic bond. Web compounds can be covalent or ionic. Web glossary summary glossary introduction learning objectives explain the formation of cations, anions, and ionic compounds predict the charge of common metallic and nonmetallic elements, and write their electron configurations describe the formation of covalent bonds define electronegativity and assess the polarity of covalent bonds

.PNG)


.PNG)

:max_bytes(150000):strip_icc()/ionic-bond-58fd4ea73df78ca1590682ad.jpg)

