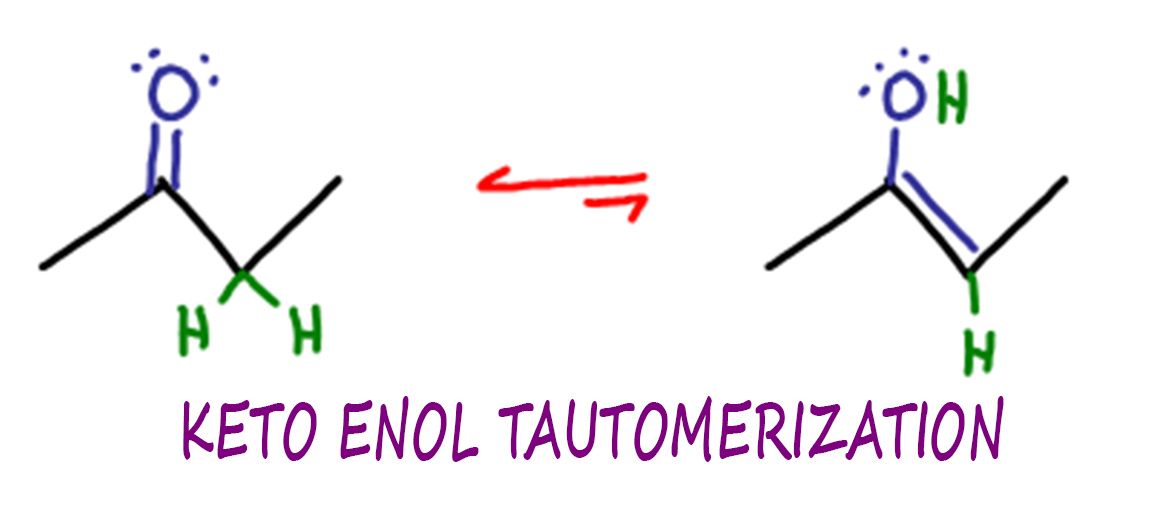
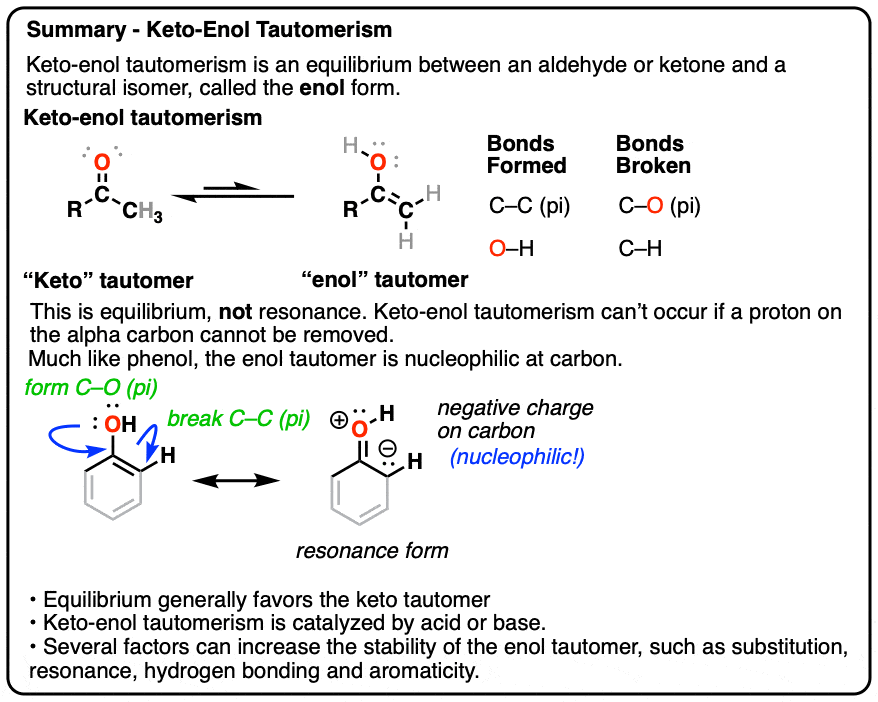
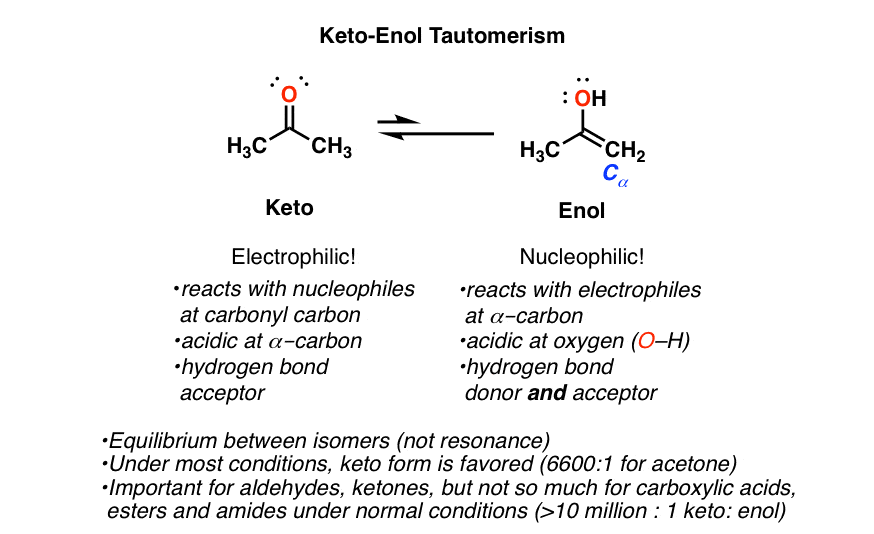
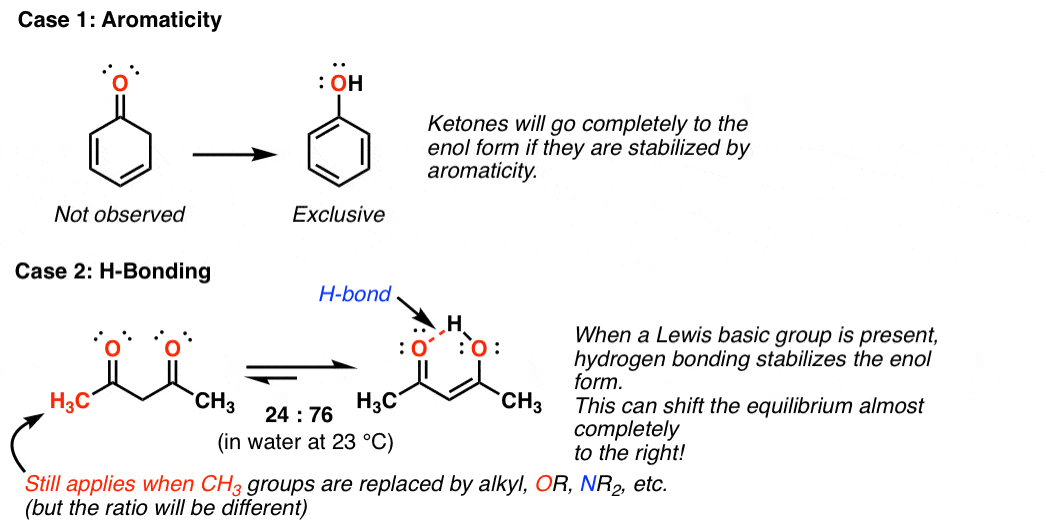
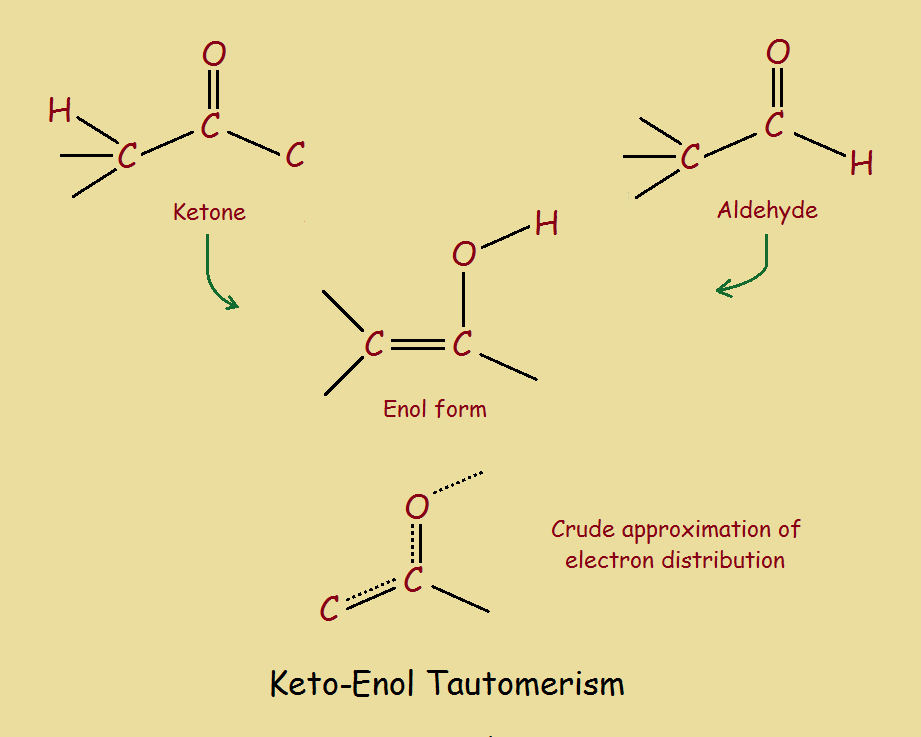
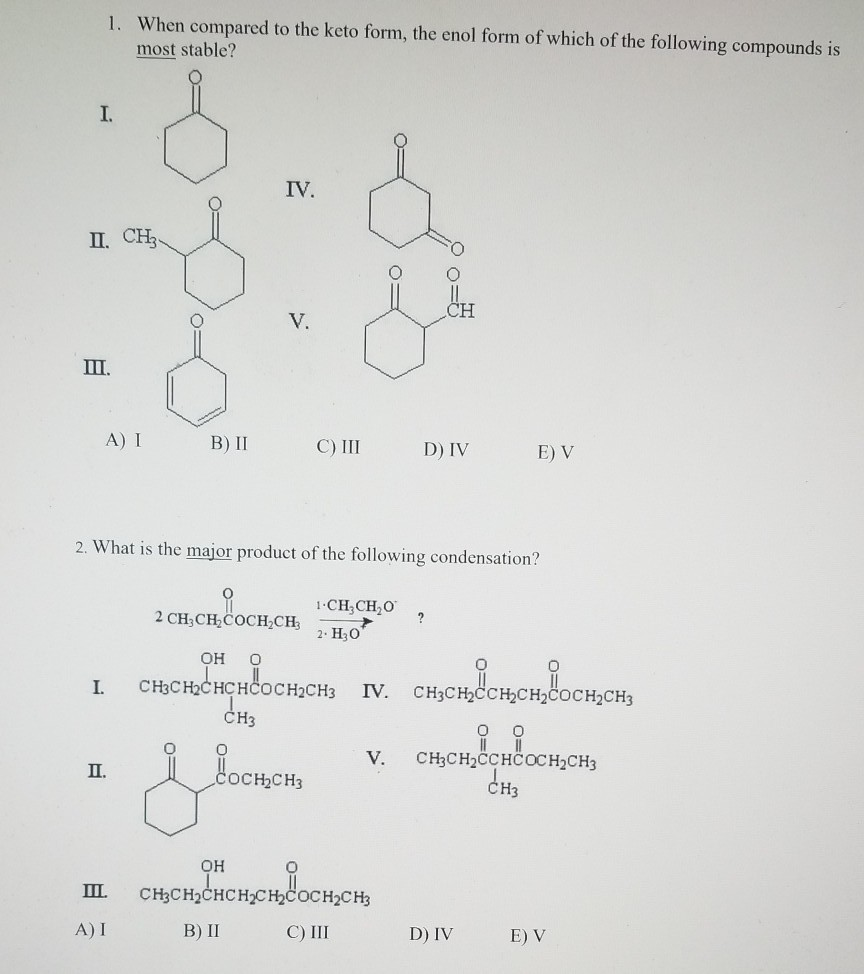
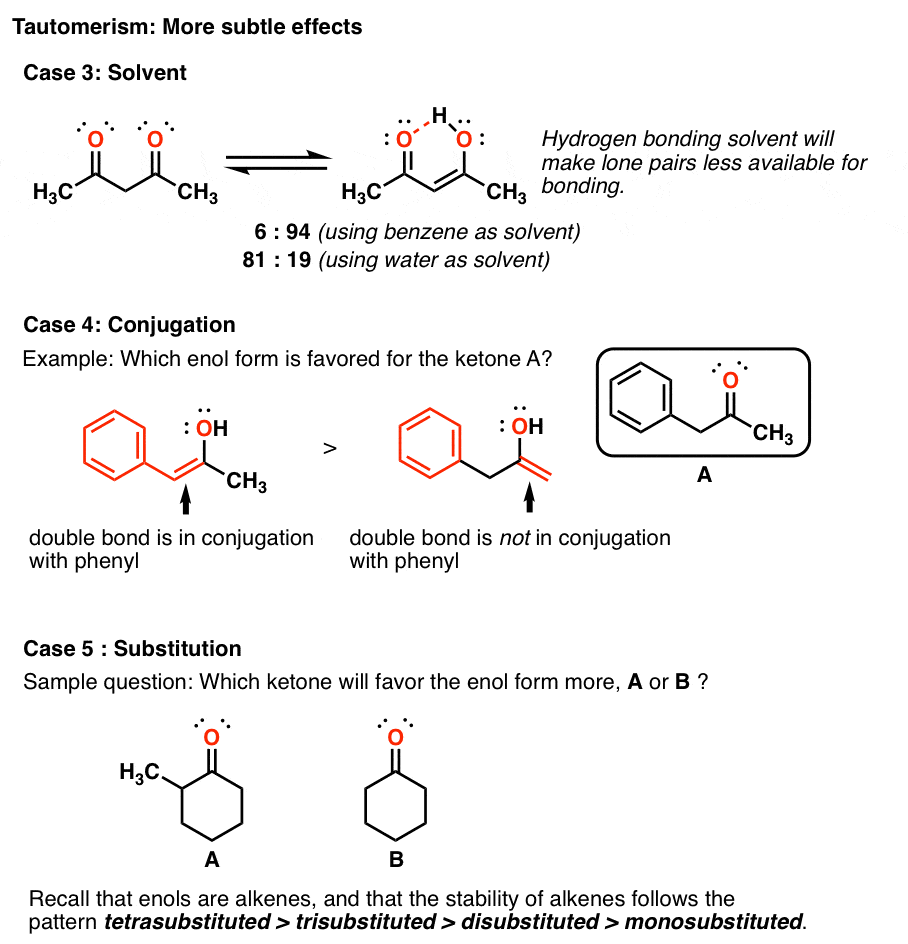
Keto Vs Enol Form
Keto Vs Enol Form - Also there is a factor that is resonance energy of c=o, since it is highly polar and may have a dipolar contributing structure as well hence its dipole moment are generally greater. Web which will be the major form among the two tautomeric forms? Regarding uracil, the first reference that comes up in a bibliographic search is this paper [2]. The keto and enol forms are therefore described as tautomers of each other. Generally, whenever keto form are stable its because of greater c=o bond energy than that if c=c. Web the detection of the separate resonances of the keto and enol forms shows that the enol and keto forms are not interconverted rapidly at room temperature, and this is in agreement with the observation that the enol and keto forms can be separated by aseptic distillation and separately preserved at low temperatures. The molecular formula does not change: On the other hand, there is c=o, with greater bond energy in the keto form. Web the s 1 state pecs reveal that the keto form is thermodynamically preferred over the enol form (fig. According to me, it should be enol form due to resonance stabilization as well has five membered ring formes througj hydrogen bonding.
According to me, it should be enol form due to resonance stabilization as well has five membered ring formes througj hydrogen bonding. Web keto vs enol bases. Web answer (1 of 19): Web the detection of the separate resonances of the keto and enol forms shows that the enol and keto forms are not interconverted rapidly at room temperature, and this is in agreement with the observation that the enol and keto forms can be separated by aseptic distillation and separately preserved at low temperatures. Thus more hyperconjugation is possible in second, hence second is more stable. Web which will be the major form among the two tautomeric forms? The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons. Of course, such stabilization is not possible for the keto form. Standard keto and rare enol forms of t & g differ by a spontaneous proton shift [an h nucleus] between the adjacent c and n molecules. Regarding uracil, the first reference that comes up in a bibliographic search is this paper [2].
Web we also notice that the most stable keto tautomer is not the same in the gas phase and in solution, and that both keto and enol have many tautomers close in free energy, showing the limits of the simple keto vs. Web we know that keto is more stable than enol tautomer so structure ii is more stable than structure i. The molecular formula does not change: Web the s 1 state pecs reveal that the keto form is thermodynamically preferred over the enol form (fig. The keto and enol forms are therefore described as tautomers of each other. Regarding uracil, the first reference that comes up in a bibliographic search is this paper [2]. Also there is a factor that is resonance energy of c=o, since it is highly polar and may have a dipolar contributing structure as well hence its dipole moment are generally greater. Standard keto and rare enol forms of t & g differ by a spontaneous proton shift [an h nucleus] between the adjacent c and n molecules. Web 1 adding to all your points, second enol form has more number of alpha hydrogens (total 8) compared to first (total 3 alpha h). The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons.
Keto Enol Tautomerization Reaction and Mechanism in Acid and Base
On the other hand, there is c=o, with greater bond energy in the keto form. The keto and enol forms are therefore described as tautomers of each other. Resonance and hydrogen bonding increases enol content. Web keto vs enol bases. The keto and enol forms are tautomers of each other.
Duck.News
Web 1 adding to all your points, second enol form has more number of alpha hydrogens (total 8) compared to first (total 3 alpha h). The molecular formula does not change: The keto and enol forms are therefore described as tautomers of each other. Web which will be the major form among the two tautomeric forms? The keto and enol.
KetoEnol Tautomerism Key Points Master Organic Chemistry
The molecular formula does not change: Web answer (1 of 19): Of course, such stabilization is not possible for the keto form. Regarding uracil, the first reference that comes up in a bibliographic search is this paper [2]. Why enol form of ethyl acetoacetate is more stable than keto form?
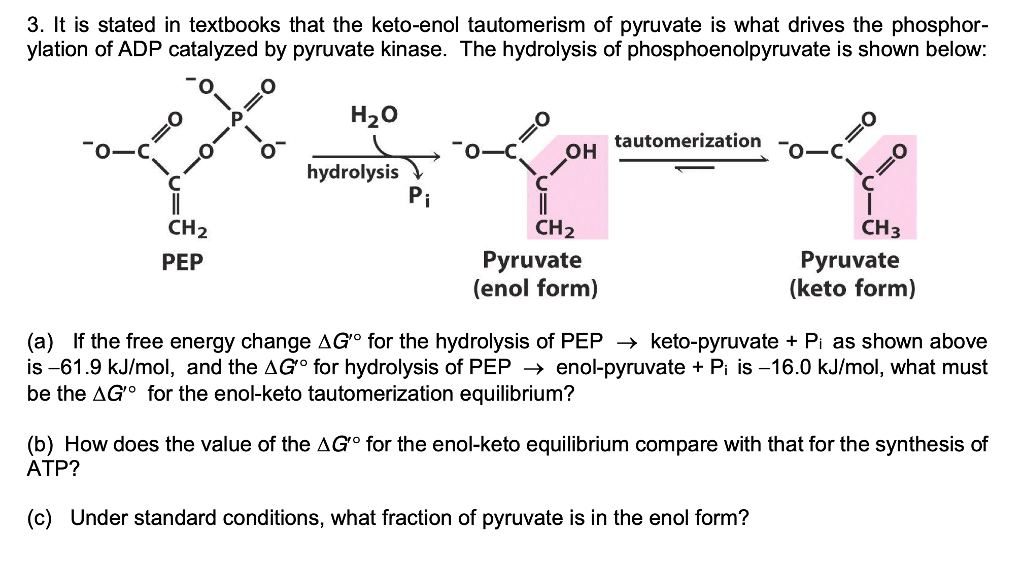
Solved 3. It is stated in textbooks that the ketoenol
Web 1 adding to all your points, second enol form has more number of alpha hydrogens (total 8) compared to first (total 3 alpha h). Why enol form of ethyl acetoacetate is more stable than keto form? On the other hand, there is c=o, with greater bond energy in the keto form. The keto and enol forms are therefore described.
Pictures of the Day
Web which will be the major form among the two tautomeric forms? Web the s 1 state pecs reveal that the keto form is thermodynamically preferred over the enol form (fig. Why enol form of ethyl acetoacetate is more stable than keto form? Web we also notice that the most stable keto tautomer is not the same in the gas.
KetoEnol Tautomerism Key Points Master Organic Chemistry
Web we know that keto is more stable than enol tautomer so structure ii is more stable than structure i. Web the s 1 state pecs reveal that the keto form is thermodynamically preferred over the enol form (fig. The keto and enol forms are therefore described as tautomers of each other. The keto and enol forms are tautomers of.
Keto Enol Tautomerism What Is It and Why Is It Important?
Web we know that keto is more stable than enol tautomer so structure ii is more stable than structure i. Standard keto and rare enol forms of t & g differ by a spontaneous proton shift [an h nucleus] between the adjacent c and n molecules. Of course, such stabilization is not possible for the keto form. According to me,.
Solved When compared to the keto form, the enol form of
Also there is a factor that is resonance energy of c=o, since it is highly polar and may have a dipolar contributing structure as well hence its dipole moment are generally greater. The keto and enol forms are therefore described as tautomers of each other. Standard keto and rare enol forms of t & g differ by a spontaneous proton.
KetoEnol Tautomerism Key Points Master Organic Chemistry
The keto and enol forms are tautomers of each other. Web 1 adding to all your points, second enol form has more number of alpha hydrogens (total 8) compared to first (total 3 alpha h). Thus more hyperconjugation is possible in second, hence second is more stable. Resonance and hydrogen bonding increases enol content. Web the detection of the separate.
organic chemistry Which is the more stable enol form? Chemistry
The molecular formula does not change: Web which will be the major form among the two tautomeric forms? Web the detection of the separate resonances of the keto and enol forms shows that the enol and keto forms are not interconverted rapidly at room temperature, and this is in agreement with the observation that the enol and keto forms can.
Why Enol Form Of Ethyl Acetoacetate Is More Stable Than Keto Form?
Resonance and hydrogen bonding increases enol content. Of course, such stabilization is not possible for the keto form. Generally, whenever keto form are stable its because of greater c=o bond energy than that if c=c. Web the detection of the separate resonances of the keto and enol forms shows that the enol and keto forms are not interconverted rapidly at room temperature, and this is in agreement with the observation that the enol and keto forms can be separated by aseptic distillation and separately preserved at low temperatures.
Regarding Uracil, The First Reference That Comes Up In A Bibliographic Search Is This Paper [2].
Web 1 adding to all your points, second enol form has more number of alpha hydrogens (total 8) compared to first (total 3 alpha h). Web the s 1 state pecs reveal that the keto form is thermodynamically preferred over the enol form (fig. Web which will be the major form among the two tautomeric forms? The molecular formula does not change:
The Keto And Enol Forms Are Tautomers Of Each Other.
The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons. Thus more hyperconjugation is possible in second, hence second is more stable. Also there is a factor that is resonance energy of c=o, since it is highly polar and may have a dipolar contributing structure as well hence its dipole moment are generally greater. On the other hand, there is c=o, with greater bond energy in the keto form.
The Keto And Enol Forms Are Therefore Described As Tautomers Of Each Other.
Standard keto and rare enol forms of t & g differ by a spontaneous proton shift [an h nucleus] between the adjacent c and n molecules. Web we also notice that the most stable keto tautomer is not the same in the gas phase and in solution, and that both keto and enol have many tautomers close in free energy, showing the limits of the simple keto vs. Web keto vs enol bases. Web answer (1 of 19):