Phosphorus Electron Configuration Long Form
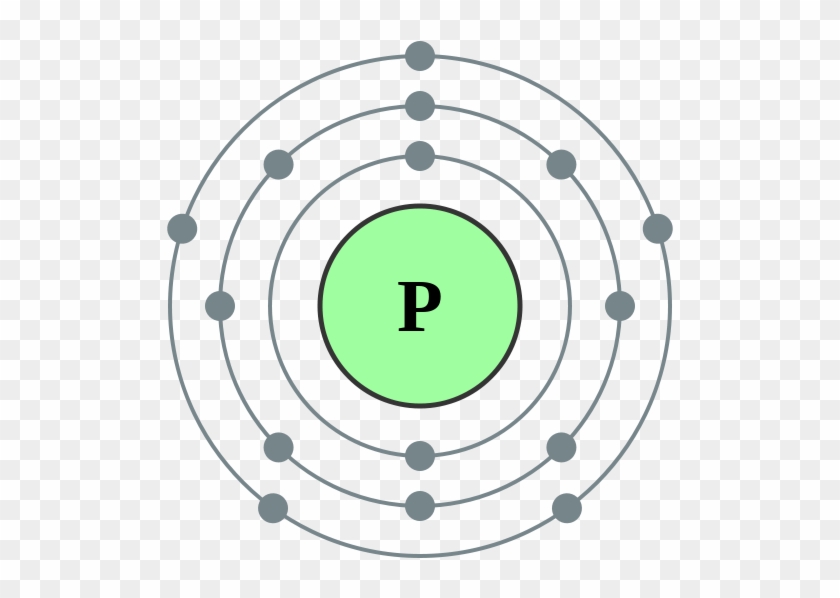
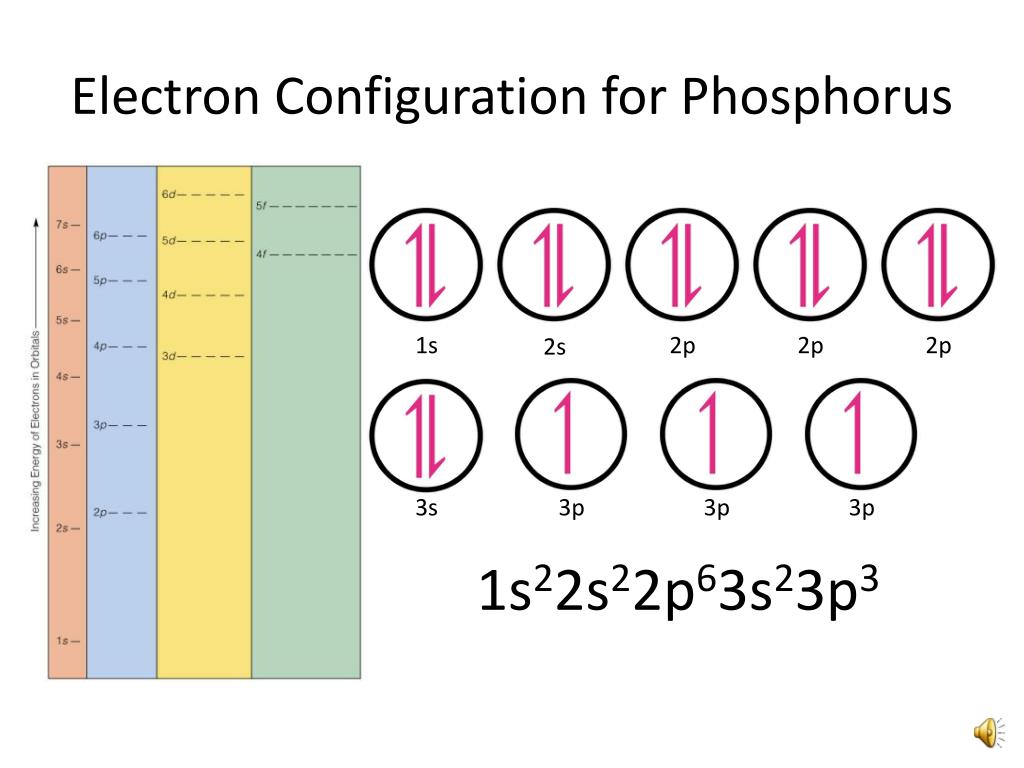
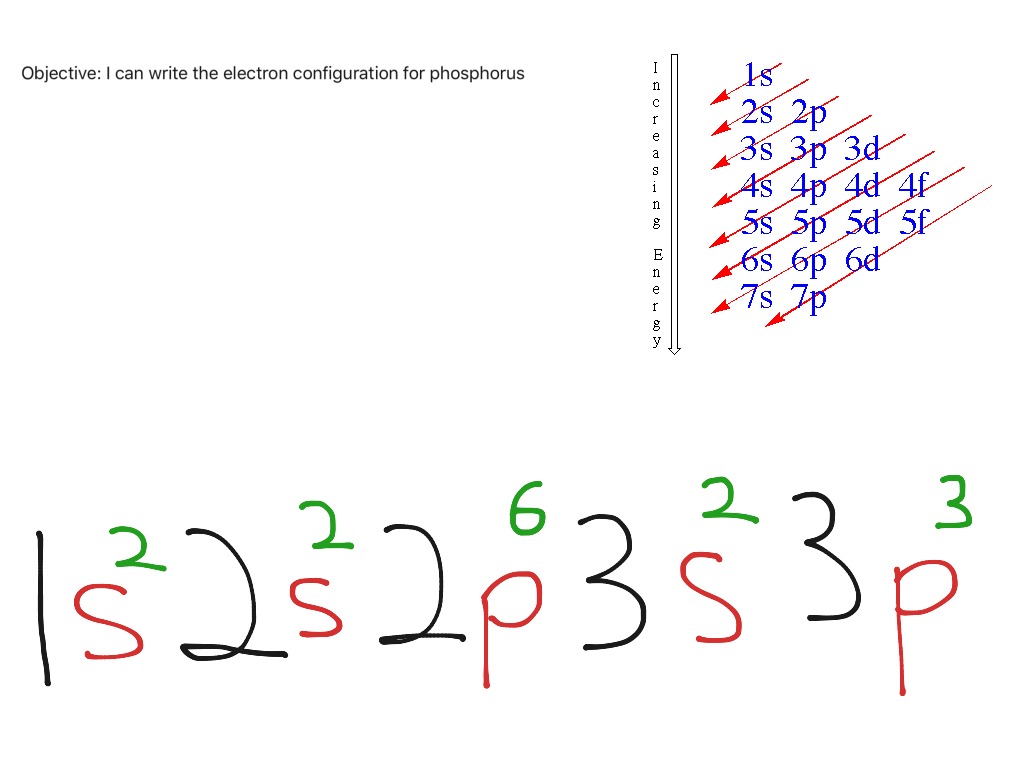
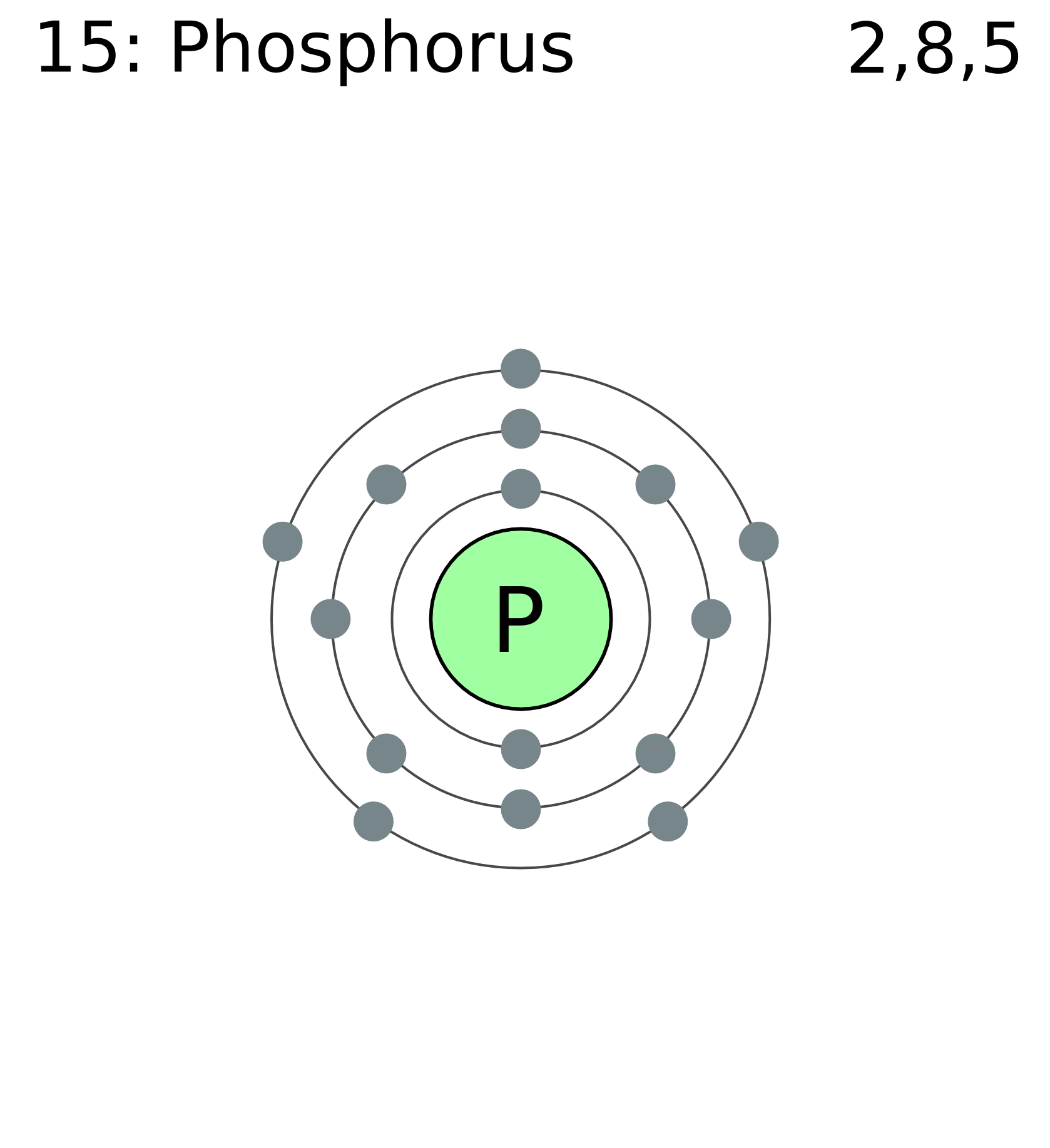
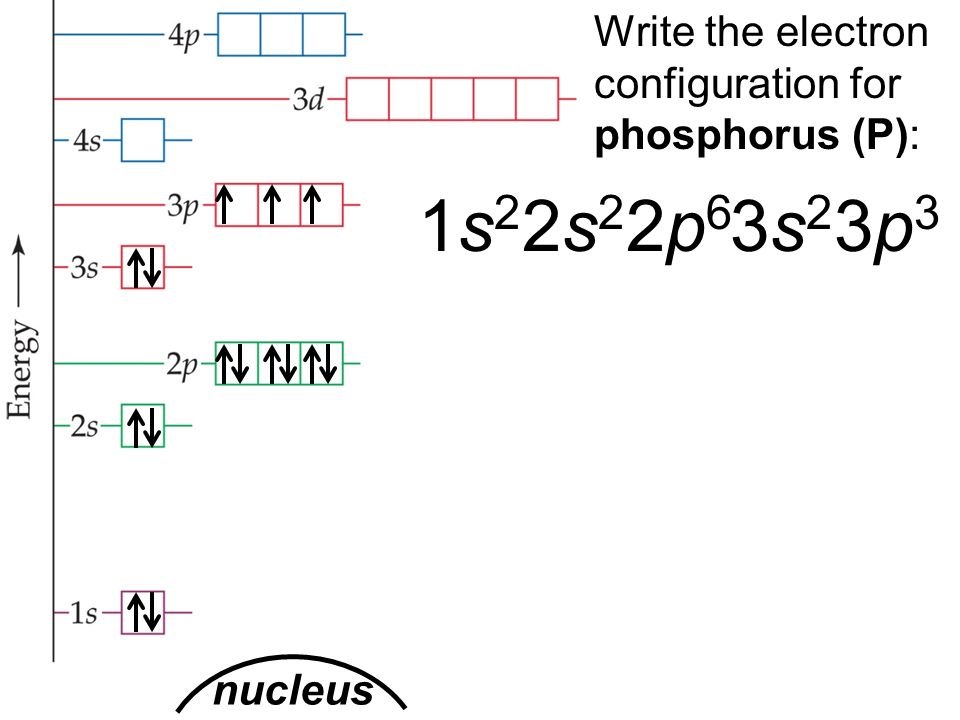
Phosphorus Electron Configuration Long Form - Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. It is in period 3 and in group 5 of the periodic table. Web electrons have a specific form of distribution (or configuration) in every atom, even phosphorus. Web in any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Expert solution step by step solved in 2 steps with 2 images It is known that elemental phosphorous occurs mostly in two forms, white and red phosphorous. When we write the configuration we'll put all 15 electrons in orbitals around the nucleus of the phosphorus atom. 553.7 k (280.5 °c, 536.9 °f) sublimation point: Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place. Web thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3.
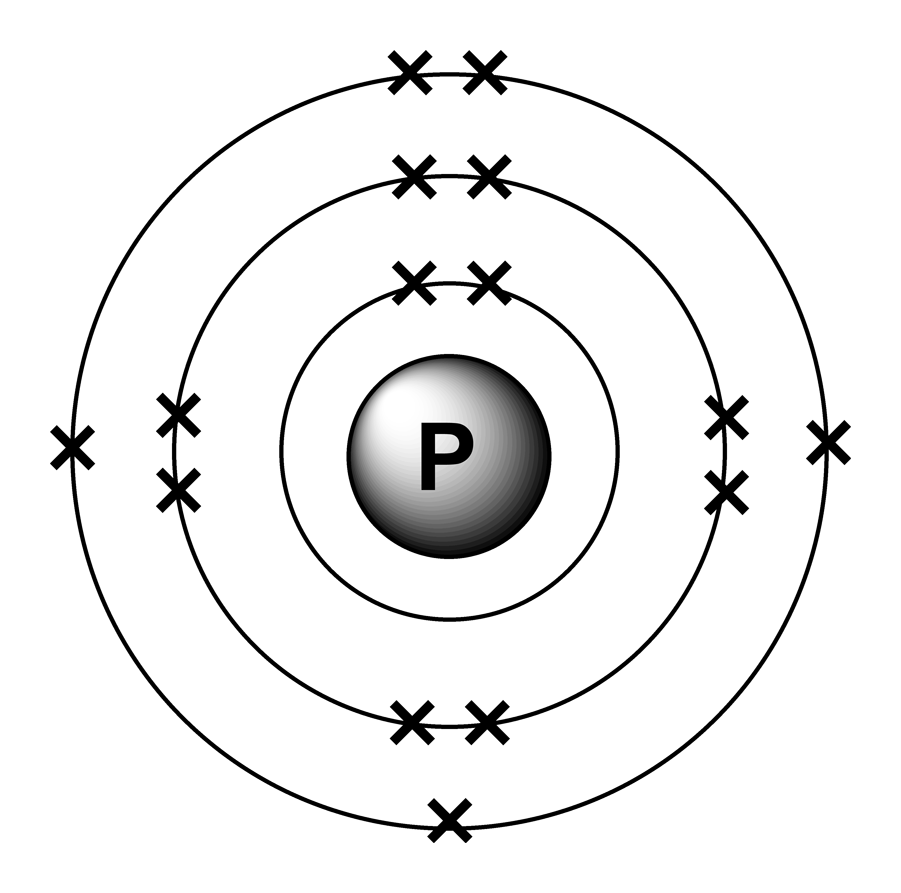
∼860 k (∼590 °c, ∼1090 °f) boiling point: Web in order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). Expert solution step by step solved in 2 steps with 2 images 1s 2 2s 2 2p 2: 2 electrons in the 3 s subshell and 3 electrons in the 3 p subshell. Web a single phosphorus atom has 15 protons and 15 electrons, but how do we know where phosphorus puts its electrons, in which orbitals? Web electron configuration of phosphorus is [ne] 3s2 3p3. Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. That gives a total of 5 valence electrons. Web electron configuration 3s 2 3p 3:
Web phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. Electronic configuration of the phosphorus atom: Web electron configuration of phosphorus is [ne] 3s2 3p3. Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. In the case of phosphorus the abbreviated electron configuration is [ne] 3s2 3p3. As an element, phosphorus exists in two major forms—white phosphorus and red phosphorus—but because it is highly reactive, phosphorus is never found as a free element on earth. This element was discovered in 1669 by hennig brand. That gives a total of 5 valence electrons. Web a single phosphorus atom has 15 protons and 15 electrons, but how do we know where phosphorus puts its electrons, in which orbitals? 1s 2 2s 2 2p 3:
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
This element was discovered in 1669 by hennig brand. Web a single phosphorus atom has 15 protons and 15 electrons, but how do we know where phosphorus puts its electrons, in which orbitals? Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. Web thus,.
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
Phosphorous is found on the earth’s crust. 1s 2 2s 2 2p 3: ∼860 k (∼590 °c, ∼1090 °f) boiling point: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Expert solution step by step solved in 2 steps with 2 images
Electron configuration example for phosphorus Science, Chemistry
The role of phosphorus is extremely significant in. Its boiling point is 550 degrees kelvin and its melting point is 317.3 degrees. When we write the configuration we'll put all 15 electrons in orbitals around the nucleus of the phosphorus atom. It is known that elemental phosphorous occurs mostly in two forms, white and red phosphorous. Web this page shows.
Phosphorus P (Element 15) of Periodic Table Elements FlashCards
Electronic configuration of chlorine atoms using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. Web based on the order of fill above, these 8 electrons would fill in.
Phosphorus Electron Configuration (P) with Orbital Diagram
Web the electron configuration of the phosphorus atom can be represented by 1s 2 2s 2 2p 6 3s 2 3p 3. The commonly used long form of the periodic table is designed to emphasize electron configurations.since it is the outermost (valence) electrons which are primarily involved in chemical interactions between atoms, the last electron added to an atom in.
a) The electron configuration of the outer layer for the phosphorus
This element was discovered in 1669 by hennig brand. Web the electronic configuration of phosphorous is 1s 2 2s 2 2p 6 3s 2 3p 3. Web phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. In this video we find the electron configuration of. 553.7 k (280.5 °c, 536.9 °f) sublimation point:
Phosphorus Electron Configuration YouTube
317.3 k (44.15 °c, 111.5 °f) red: Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. Expert solution step by step solved in 2 steps with 2 images Web thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p.
Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
In this video we find the electron configuration of. Web electrons have a specific form of distribution (or configuration) in every atom, even phosphorus. 2 electrons in the 3 s subshell and 3 electrons in the 3 p subshell. As an element, phosphorus exists in two major forms—white phosphorus and red phosphorus—but because it is highly reactive, phosphorus is never.
Load Wiring Carbon Molecular Orbital Diagram
Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. This element was discovered in 1669 by hennig brand. Web this page shows the electron configurations of the neutral gaseous atoms.
Electron configurations
Web electron configuration of beryllium (be) [he] 2s 2: Web electrons have a specific form of distribution (or configuration) in every atom, even phosphorus. Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. P (phosphorus) is an element with position number 15 in the.
Electron Configuration Of Carbon (C) [He] 2S 2 2P 2:
The distribution of electrons are as 2 electrons in 1s subshell, electrons in 2s subshell,6 electrons in 2p subshell, 2 electrons in 3s subshell and the remaining 3 electrons in 3p subshell. P (phosphorus) is an element with position number 15 in the periodic table. Web phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. This element was discovered in 1669 by hennig brand.
As An Element, Phosphorus Exists In Two Major Forms—White Phosphorus And Red Phosphorus—But Because It Is Highly Reactive, Phosphorus Is Never Found As A Free Element On Earth.
Group 15 elements are also known as pnictogens. Web the element phosphorus has 15 electrons, represented by 15p. 1s 2 2s 2 2p 2: Write the long form electron configuration for phosphorous.
Web In Order To Write The Phosphorus Electron Configuration We First Need To Know The Number Of Electrons For The P Atom (There Are 15 Electrons).
553.7 k (280.5 °c, 536.9 °f) sublimation point: Web thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. So oxygen's electron configuration would be o 1s 2 2s 2 2p 4. Web the electron configuration of the phosphorus atom can be represented by 1s 2 2s 2 2p 6 3s 2 3p 3.
In Order To Write The P Electron Configuration We First Need To Know.
Its boiling point is 550 degrees kelvin and its melting point is 317.3 degrees. Electronic configuration of the phosphorus atom: Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: