Two Point Form Of Arrhenius Equation
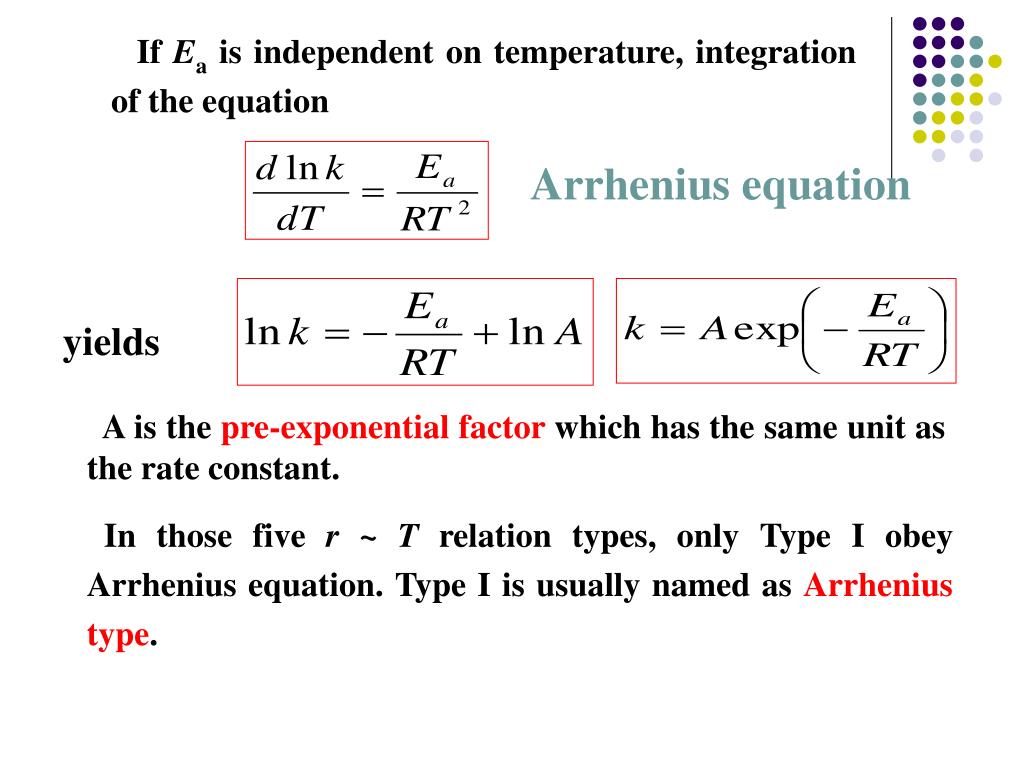
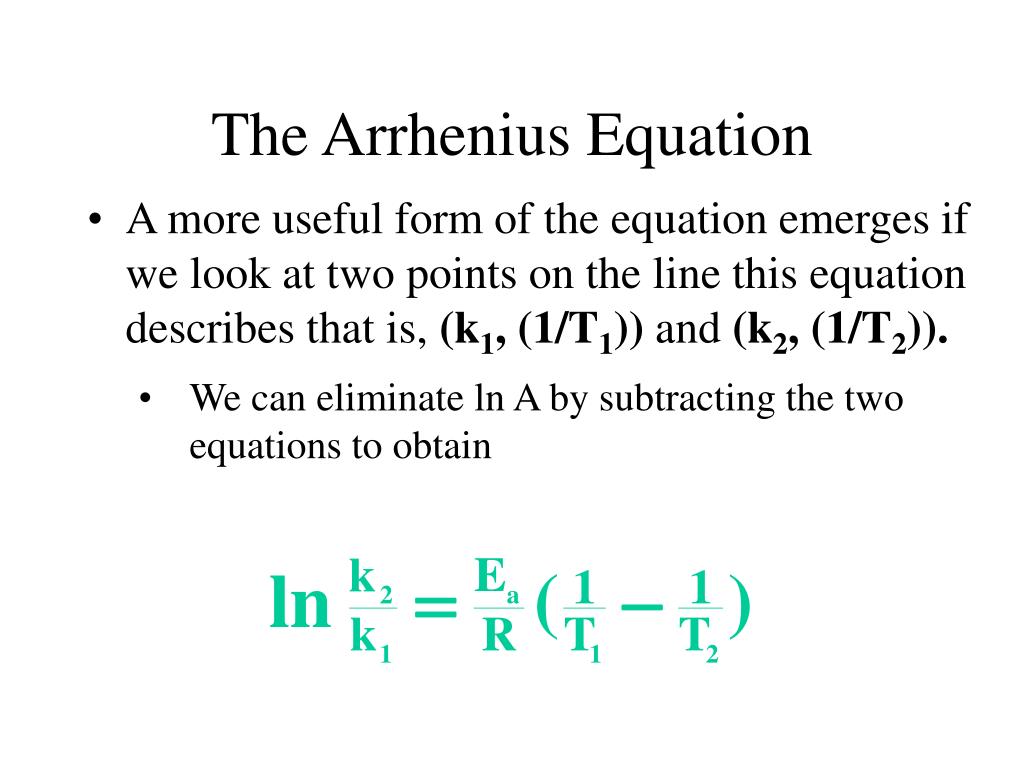
Two Point Form Of Arrhenius Equation - Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. Web two point arrhenius equation. Web the arrhenius equation, k = ae − ea / rt. Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: (6.2.3.4.2) ln k = − e a r t + ln a Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. For a second order reaction (of the form:
Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works: Subtracting equation (i) from the equation (ii), we get. Web the arrhenius equation, k = ae − ea / rt. For a second order reaction (of the form: Ea show steps k1 show steps k2 show steps t1 show steps t2. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: Web two point arrhenius equation. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants.
Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. (6.2.3.4.2) ln k = − e a r t + ln a Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. Subtracting equation (i) from the equation (ii), we get. Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. For a second order reaction (of the form: Web two point arrhenius equation. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works:
Chapter13 chemical
At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: Web two point arrhenius equation. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. (6.2.3.4.2).
Chapter13 chemical
(6.2.3.4.2) ln k = − e a r t + ln a Web two point arrhenius equation. For a second order reaction (of the form: Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to.
PPT temperaturedependence of reaction rate Arrhenius equation
(6.2.3.4.2) ln k = − e a r t + ln a Ea show steps k1 show steps k2 show steps t1 show steps t2. Web two point arrhenius equation. Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation.
13.4 The Arrhenius Equation YouTube
Web two point arrhenius equation. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: Subtracting.
PPT Halflife PowerPoint Presentation, free download ID1826480
For a second order reaction (of the form: Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. Web two point arrhenius equation. At two different temperatures t 1 and t.
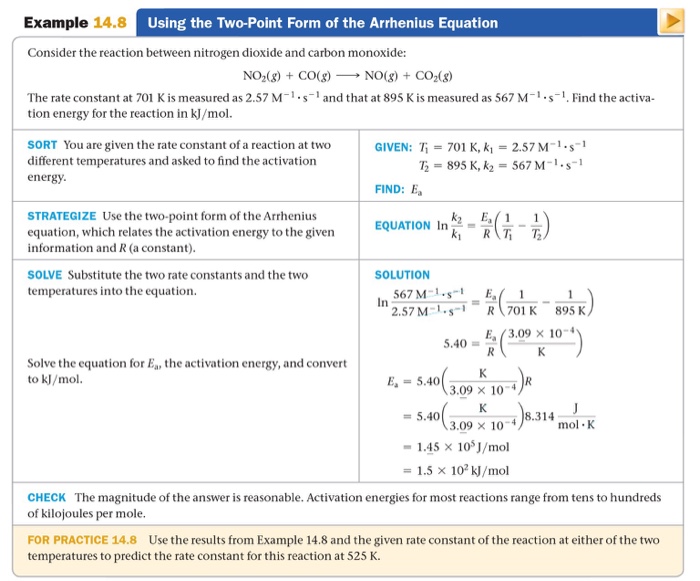
I Need Help With For Practice 14.8. I'm Not Sure H...
Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. At two different temperatures t 1 and t 2, the corresponding.
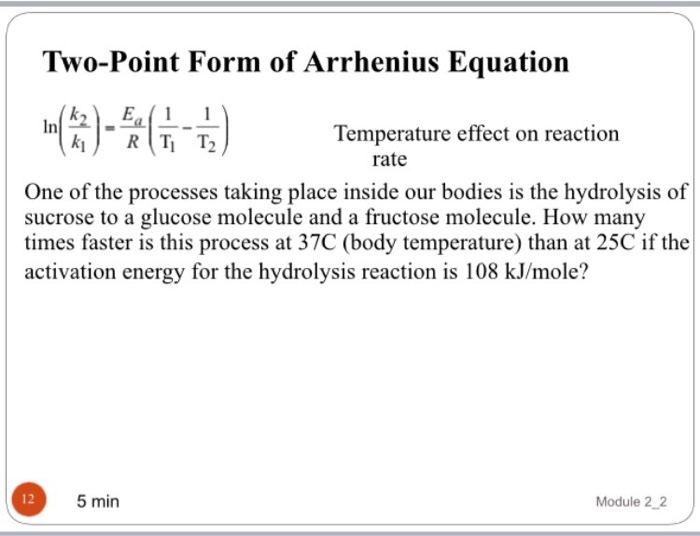
Two point arrhenius equation, Easy derivation, 3 application
Ea show steps k1 show steps k2 show steps t1 show steps t2. Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna. Web the arrhenius equation, k = ae − ea / rt. Amanda balcerzak's chemistry headquarters 136 subscribers this video shows how to. Web the arrhenius.
arrhenius equation Yahoo Image Search Results Gas constant
Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. Web two point arrhenius equation. Subtracting equation (i) from the equation (ii), we get. Web the arrhenius activation energy for two.
TwoPoint Form for Arrhenius Equation YouTube
Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. For a second order reaction (of the form: Subtracting equation (i) from the equation (ii), we get. Web the arrhenius equation, k = ae − ea / rt. Web the arrhenius activation energy for two temperature calculator.
Solved TwoPoint Form of Arrhenius Equation In N2.
Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. Subtracting equation (i) from the equation (ii), we get. Web the arrhenius equation, k = ae − ea / rt. Lnk = ln(ae − ea / rt) = lna + ln(e − ea /.
Web The Arrhenius Equation, K = Ae − Ea / Rt.
Web k = a e − e a r t arrhenius equation per molecule k = a e − e a k t linearized arrhenius equation the arrhenius equation (equation 6.2.3.4.1) can be rearranged to deal with specific situations. Web the arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. Web the arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. Lnk = ln(ae − ea / rt) = lna + ln(e − ea / rt) = (− ea r)(1 t) + lna.
Ea Show Steps K1 Show Steps K2 Show Steps T1 Show Steps T2.
For a second order reaction (of the form: At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can write as: Web two point arrhenius equation. If we look at the equation that this arrhenius equation calculator uses, we can try to understand how it works:
Amanda Balcerzak's Chemistry Headquarters 136 Subscribers This Video Shows How To.
Subtracting equation (i) from the equation (ii), we get. (6.2.3.4.2) ln k = − e a r t + ln a